Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis
- PMID: 28440858
- PMCID: PMC6478290
- DOI: 10.1002/14651858.CD012200.pub2
Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis
Abstract
Background: The treatment of multiple sclerosis has changed over the last 20 years. The advent of disease-modifying drugs in the mid-1990s heralded a period of rapid progress in the understanding and management of multiple sclerosis. With the support of magnetic resonance imaging early diagnosis is possible, enabling treatment initiation at the time of the first clinical attack. As most of the disease-modifying drugs are associated with adverse events, patients and clinicians need to weigh the benefit and safety of the various early treatment options before taking informed decisions.
Objectives: 1. to estimate the benefit and safety of disease-modifying drugs that have been evaluated in all studies (randomised or non-randomised) for the treatment of a first clinical attack suggestive of MS compared either with placebo or no treatment;2. to assess the relative efficacy and safety of disease-modifying drugs according to their benefit and safety;3. to estimate the benefit and safety of disease-modifying drugs that have been evaluated in all studies (randomised or non-randomised) for treatment started after a first attack ('early treatment') compared with treatment started after a second attack or at another later time point ('delayed treatment').
Search methods: We searched the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group Trials Register, MEDLINE, Embase, CINAHL, LILACS, clinicaltrials.gov, the WHO trials registry, and US Food and Drug Administration (FDA) reports, and searched for unpublished studies (until December 2016).
Selection criteria: We included randomised and observational studies that evaluated one or more drugs as monotherapy in adult participants with a first clinical attack suggestive of MS. We considered evidence on alemtuzumab, azathioprine, cladribine, daclizumab, dimethyl fumarate, fingolimod, glatiramer acetate, immunoglobulins, interferon beta-1b, interferon beta-1a (Rebif®, Avonex®), laquinimod, mitoxantrone, natalizumab, ocrelizumab, pegylated interferon beta-1a, rituximab and teriflunomide.
Data collection and analysis: Two teams of three authors each independently selected studies and extracted data. The primary outcomes were disability-worsening, relapses, occurrence of at least one serious adverse event (AE) and withdrawing from the study or discontinuing the drug because of AEs. Time to conversion to clinically definite MS (CDMS) defined by Poser diagnostic criteria, and probability to discontinue the treatment or dropout for any reason were recorded as secondary outcomes. We synthesized study data using random-effects meta-analyses and performed indirect comparisons between drugs. We calculated odds ratios (OR) and hazard ratios (HR) along with relative 95% confidence intervals (CI) for all outcomes. We estimated the absolute effects only for primary outcomes. We evaluated the credibility of the evidence using the GRADE system.
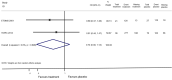
Main results: We included 10 randomised trials, eight open-label extension studies (OLEs) and four cohort studies published between 2010 and 2016. The overall risk of bias was high and the reporting of AEs was scarce. The quality of the evidence associated with the results ranges from low to very low. Early treatment versus placebo during the first 24 months' follow-upThere was a small, non-significant advantage of early treatment compared with placebo in disability-worsening (6.4% fewer (13.9 fewer to 3 more) participants with disability-worsening with interferon beta-1a (Rebif®) or teriflunomide) and in relapses (10% fewer (20.3 fewer to 2.8 more) participants with relapses with teriflunomide). Early treatment was associated with 1.6% fewer participants with at least one serious AE (3 fewer to 0.2 more). Participants on early treatment were on average 4.6% times (0.3 fewer to 15.4 more) more likely to withdraw from the study due to AEs. This result was mostly driven by studies on interferon beta 1-b, glatiramer acetate and cladribine that were associated with significantly more withdrawals for AEs. Early treatment decreased the hazard of conversion to CDMS (HR 0.53, 95% CI 0.47 to 0.60). Comparing active interventions during the first 24 months' follow-upIndirect comparison of interferon beta-1a (Rebif®) with teriflunomide did not show any difference on reducing disability-worsening (OR 0.84, 95% CI 0.43 to 1.66). We found no differences between the included drugs with respect to the hazard of conversion to CDMS. Interferon beta-1a (Rebif®) and teriflunomide were associated with fewer dropouts because of AEs compared with interferon beta-1b, cladribine and glatiramer acetate (ORs range between 0.03 and 0.29, with substantial uncertainty). Early versus delayed treatmentWe did not find evidence of differences between early and delayed treatments for disability-worsening at a maximum of five years' follow-up (3% fewer participants with early treatment (15 fewer to 11.1 more)). There was important variability across interventions; early treatment with interferon beta-1b considerably reduced the odds of participants with disability-worsening during three and five years' follow-up (OR 0.52, 95% CI 0.32 to 0.84 and OR 0.57, 95% CI 0.36 to 0.89). The early treatment group had 19.6% fewer participants with relapses (26.7 fewer to 12.7 fewer) compared to late treatment at a maximum of five years' follow-up and early treatment decreased the hazard of conversion to CDMS at any follow-up up to 10 years (i.e. over five years' follow-up HR 0.62, 95% CI 0.53 to 0.73). We did not draw any conclusions on long-term serious AEs or discontinuation due to AEs because of inadequacies in the available data both in the included OLEs and cohort studies.
Authors' conclusions: Very low-quality evidence suggests a small and uncertain benefit with early treatment compared with placebo in reducing disability-worsening and relapses. The advantage of early treatment compared with delayed on disability-worsening was heterogeneous depending on the actual drug used and based on very low-quality evidence. Low-quality evidence suggests that the chances of relapse are less with early treatment compared with delayed. Early treatment reduced the hazard of conversion to CDMS compared either with placebo, no treatment or delayed treatment, both in short- and long-term follow-up. Low-quality evidence suggests that early treatment is associated with fewer participants with at least one serious AE compared with placebo. Very low-quality evidence suggests that, compared with placebo, early treatment leads to more withdrawals or treatment discontinuation due to AEs. Difference between drugs on short-term benefit and safety was uncertain because few studies and only indirect comparisons were available. Long-term safety of early treatment is uncertain because of inadequately reported or unavailable data.
Conflict of interest statement
GF ‐ none. As Co‐ordinating Editor, Dr. Filippini was excluded from the editorial process to ensure separation of the review author from the editorial process. This included all editorial decisions and related activities (e.g. sign‐off for publication). CDG ‐ received financial support for conducting the review process from a grant financed by Swiss MS Register. This had no bearing on, and did not influence, what has been written in the submitted work. MC ‐ received personal compensation from Merck, Biogen, Novartis and Sanofi‐Genzyme for serving on advisory boards and for providing expert testimony as well as for travel/ accommodation/meeting expenses. Dr. Clerico's institution received some grants for research projects from Merck. OB ‐ received salary from Cognizant Technology Solutions for epidemiological consultation for Pharma companies. MM ‐ speaker honoraria from Merck Serono and Novartis; received financial support for travel/accommodation/meeting expenses from Biogen Idec, Novartis, Genzyme and Teva. This had no bearing on, and did not influence, what has been written in the submitted work. FP ‐ none SF ‐ received honoraria for consultancy, educational activities and/or lectures from Allergan, Bayer, Biogen, Genzyme, Merck, Novartis, Sanofi and Teva. IT ‐ none AS ‐ none GS ‐ none
Figures












Update of
- doi: 10.1002/14651858.CD012200
Similar articles
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article. Review.
-
Adverse effects of immunotherapies for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 30;11(11):CD012186. doi: 10.1002/14651858.CD012186.pub2. Cochrane Database Syst Rev. 2023. PMID: 38032059 Review.
-
Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2013 Jun 6;(6):CD008933. doi: 10.1002/14651858.CD008933.pub2. Cochrane Database Syst Rev. 2013. PMID: 23744561 Review.
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2016 Mar 22;3(3):CD009882. doi: 10.1002/14651858.CD009882.pub3. Cochrane Database Syst Rev. 2016. PMID: 27003123 Free PMC article. Review.
-
Immunomodulators and immunosuppressants for progressive multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Sep 10;9(9):CD015443. doi: 10.1002/14651858.CD015443.pub2. Cochrane Database Syst Rev. 2024. PMID: 39254048 Free PMC article.
Cited by
-
Risk of cancer development associated with disease-modifying therapies for multiple sclerosis: study protocol for a systematic review and meta-analysis of randomised and non-randomised studies.Syst Rev. 2024 Oct 18;13(1):263. doi: 10.1186/s13643-024-02677-z. Syst Rev. 2024. PMID: 39425150 Free PMC article.
-
Treatment Effects in Randomized and Nonrandomized Studies of Pharmacological Interventions: A Meta-Analysis.JAMA Netw Open. 2024 Sep 3;7(9):e2436230. doi: 10.1001/jamanetworkopen.2024.36230. JAMA Netw Open. 2024. PMID: 39331390 Free PMC article.
-
New views on the complex interplay between degeneration and autoimmunity in multiple sclerosis.Front Cell Neurosci. 2024 Aug 5;18:1426231. doi: 10.3389/fncel.2024.1426231. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39161786 Free PMC article.
-
Disease-modifying therapy initiation patterns in multiple sclerosis in three large MS populations.Ther Adv Neurol Disord. 2024 Mar 15;17:17562864241233044. doi: 10.1177/17562864241233044. eCollection 2024. Ther Adv Neurol Disord. 2024. PMID: 38495364 Free PMC article.
-
Disease-modifying drugs, multiple sclerosis and infection-related healthcare use in British Columbia, Canada: a population-based study.Lancet Reg Health Am. 2024 Jan 6;29:100667. doi: 10.1016/j.lana.2023.100667. eCollection 2024 Jan. Lancet Reg Health Am. 2024. PMID: 38269206 Free PMC article.
References
References to studies included in this review
Achiron 2004 {published data only}
-
- Achiron A, Kishner I, Sarova‐Pinhas I, Raz H, Faibel M, Stern Y, et al. Intravenous immunoglobulin treatment following the first demyelinating event suggestive of multiple sclerosis: a randomized, double‐blind, placebo‐controlled trial. Archives of Neurology 2004;61(10):1515‐20. [PUBMED: 15477504] - PubMed
ACISS 2010 {published data only}
-
- Fazekas F, Baumhackl U, Berger T, Deisenhammer F, Fuchs S, Kristoferitsch W, et al. Decision‐making for and impact of early immunomodulatory treatment: the Austrian Clinically Isolated Syndrome Study (ACISS). European Journal of Neurology 2010;17(6):852‐60. [PUBMED: 20100231] - PubMed
BENEFIT 2006 {published data only}
-
- Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, et al. Treatment with interferon beta‐1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006;67(7):1242‐9. [PUBMED: 16914693] - PubMed
BENEFIT 2007 (3 years FU) {published data only}
-
- Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, et al. Effect of early versus delayed interferon beta‐1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3‐year follow‐up analysis of the BENEFIT study. Lancet 2007;370(9585):389‐97. [PUBMED: 17679016] - PubMed
BENEFIT 2009 (5 years FU) {published data only}
-
- Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, et al. Long‐term effect of early treatment with interferon beta‐1bafter a first clinical event suggestive of multiple sclerosis: 5‐year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurology 2009;8(11):987–97. [PUBMED: 19748319] - PubMed
BENEFIT 2014 (8.7 years FU) {published data only}
BENEFIT 2016 (11 years FU) {published data only}
CHAMPS 2000 {published data only}
-
- CHAMPS Study Group. Baseline MRI characteristics of patients at high risk for multiple sclerosis: results from the CHAMPS trial. Controlled High‐Risk Subjects Avonex Multiple Sclerosis Prevention Study. Multiple Sclerosis 2002;8(4):330‐8. [PUBMED: 12166504] - PubMed
-
- Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, et al. Intramuscular interferon beta‐1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. New England Journal of Medicine 2000;343(13):898‐904. [PUBMED: 11006365] - PubMed
CHAMPS 2006 (5 years FU) {published data only}
-
- Kinkel RP, Kollman C, O'Connor P, Murray TJ, Simon J, Arnold D, et al. IM interferon beta‐1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology 2006;66(5):678‐84. [PUBMED: 16436649] - PubMed
CHAMPS 2012 (10 years FU) {published data only}
-
- Kinkel RP, Dontchev M, Kollman C, Skaramagas TT, O'Connor PW, Simon JH, et al. Association between immediate initiation of intramuscular interferon beta‐1a at the time of a clinically isolated syndrome and long‐term outcomes: a 10‐year follow‐up of the Controlled High‐Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. Archives of Neurology 2012;69(2):183‐90. [PUBMED: 21987393] - PubMed
-
- Simon JH, Kinkel RP, Kollman C, O'Connor P, Fisher E, You X, et al. Ten‐year follow‐up of the 'minimal MRI lesion' subgroup from the original CHAMPS Multiple Sclerosis Prevention Trial. Multiple Sclerosis 2015;21(4):415‐22. [PUBMED: 25344370] - PubMed
ETOMS 2001 {published data only}
-
- Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernández O, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 2001;357(9268):1576‐82. [PUBMED: 11377645] - PubMed
GERONIMUS 2013 {published data only}
-
- D'Alessandro R, Vignatelli L, Lugaresi A, Baldin E, Granella F, Tola MR, et al. Risk of multiple sclerosis following clinically isolated syndrome: a 4‐year prospective study. Journal of Neurology 2013;260(6):1583‐93. [PUBMED: 23377434] - PubMed
Motamed 2007 {published data only}
-
- Motamed MR, Najimi N, Fereshtehnejad SM. The effect of interferon‐beta‐1a on relapses and progression of disability in patients with clinically isolated syndromes (CIS) suggestive of multiple sclerosis. Clinical Neurology and Neurosurgery 2007;109(4):344‐9. [PUBMED: 17300863] - PubMed
MSBASIS 2016 {published data only}
-
- Spelman T, Meyniel C, Rojas JI, Lugaresi A, Izquierdo G, Grand'Maison F, et al. Quantifying risk of early relapse in patients with first demyelinating events: prediction in clinical practice. Multiple Sclerosis Journal 2016;1:1‐12. [PUBMED: 27885062] - PubMed
ORACLE 2014 {published data only}
-
- Leist TP, Comi G, Cree BA, Coyle PK, Freedman MS, Hartung HP, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurology 2014;13(3):257‐67. [PUBMED: 24502830] - PubMed
Pakdaman 2007 {published data only}
-
- Pakdaman H, Sahraian MA, Fallah A, Pakdaman R, Ghareghozli K, Ghafarpour M, et al. Effect of early interferon beta‐1a therapy on conversion to multiple sclerosis in Iranian patients with a first demyelinating event. Acta Neurologica Scandinavica 2007;115(6):429‐31. [PUBMED: 17511854] - PubMed
PRECISE 2009 {published data only}
-
- Comi G, Martinelli V, Rodegher M, Moiola L, Bajenaru O, Carra A, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double‐blind, placebo‐controlled trial. Lancet 2009;374(9700):1503‐11. [PUBMED: 19815268] - PubMed
PRECISE 2013 (5 years FU) {published data only}
-
- Comi G, Martinelli V, Rodegher M, Moiola L, Leocani L, Bajenaru O, et al. Effects of early treatment with glatiramer acetate in patients with clinically isolated syndrome. Multiple Sclerosis 2013;19(8):1074‐83. [PUBMED: 23234810] - PubMed
REFLEX 2012 {published data only}
-
- Comi G, Stefano N, Freedman MS, Barkhof F, Polman CH, Uitdehaag BM, et al. Comparison of two dosing frequencies of subcutaneous interferon beta‐1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurology 2012;11(1):33‐41. [PUBMED: 22146409] - PubMed
REFLEX 2016 (3 and 5 years FU) {published data only}
-
- Comi G, Stefano N, Freedman M, Barkhof F, Uitdehaag B, Vos M, et al. Subcutaneous interferon β‐1a in the treatment of clinically isolated syndromes: 3‐year and 5‐year results of the phase III dosing frequency‐blind multicentre REFLEXION study. Journal of Neurology, Neurosurgery, and Psychiatry 2016;88(4):285‐94. [PUBMED: 28039317] - PubMed
Tintore 2015 {published data only}
-
- Tintore M, Rovira À, Río J, Otero‐Romero S, Arrambide G, Tur C, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015;138:1863‐74. [PUBMED: 25902415] - PubMed
TOPIC 2014 {published data only}
-
- Miller AE, Wolinsky JS, Kappos L, Comi G, Freedman MS, Olsson TP, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurology 2014;13(10):977‐86. [PUBMED: 25192851] - PubMed
References to studies excluded from this review
BENEFIT 2007 {published data only}
-
- Barkhof F, Polman CH, Radue EW, Kappos L, Freedman MS, Edan G, et al. Magnetic resonance imaging effects of interferon beta‐1b in the BENEFIT study: integrated 2‐year results. Archives of Neurology 2007;64(9):1292‐8. [PUBMED: 17846268] - PubMed
BENEFIT 2008 {published data only}
-
- Polman C, Kappos L, Freedman MS, Edan G, Hartung HP, Miller DH, et al. Subgroups of the BENEFIT study: risk of developing MS and treatment effect of interferon beta‐1b. Journal of Neurology 2008;255(4):480‐7. [PUBMED: 18004635] - PubMed
BENEFIT 2011 {published data only}
-
- Hartung HP, Freedman MS, Polman CH, Edan G, Kappos L, Miller DH, et al. Interferon β‐1b‐neutralizing antibodies 5 years after clinically isolated syndrome. Neurology 2011;77(9):835‐43. [PUBMED: 21849647] - PubMed
BENEFIT 2012 {published data only}
BENEFIT 2014a {published data only}
BENEFIT 2014b {published data only}
-
- Nagtegaal GJ, Pohl C, Wattjes MP, Hulst HE, Freedman MS, Hartung HP, et al. Interferon beta‐1b reduces black holes in a randomised trial of clinically isolated syndrome. Multiple Sclerosis 2014;20(2):234‐42. [PUBMED: 23842212] - PubMed
CHAMPIONS 2015 {published data only}
-
- Simon JH, Kinkel RP, Kollman C, O'Connor P, Fisher E, You X, et al. Ten‐year follow‐up of the 'minimal MRI lesion' subgroup from the original CHAMPS Multiple Sclerosis Prevention Trial. Multiple Sclerosis 2015;21(4):415‐22. [PUBMED: 25344370 ] - PubMed
CHAMPS 2001 {published data only}
-
- CHAMPS Study Group. Interferon ‐1a for optic neuritis patients at high risk for multiple sclerosis. American Journal of Ophthalmology 2001;132(4):463‐71. [PUBMED: 11589865] - PubMed
CHAMPS 2002a {published data only}
-
- CHAMPS Study Group. Predictors of short‐term disease activity following a first clinical demyelinating event: analysis of the CHAMPS placebo group. Multiple Sclerosis 2002;8(5):405‐9. [PUBMED: 12356207] - PubMed
CHAMPS 2002b {published data only}
-
- Beck RW, Chandler DL, Cole SR, Simon JH, Jacobs LD, Kinkel RP, et al. Interferon beta‐1a for early multiple sclerosis: CHAMPS trial subgroup analyses. Annals of Neurology 2002;51(4):481‐90. [PUBMED: 11921054] - PubMed
CHAMPS 2002c {published data only}
-
- CHAMPS Study Group. Baseline MRI characteristics of patients at high risk for multiple sclerosis: results from the CHAMPS trial. Multiple Sclerosis 2002;8(4):330‐8. [PUBMED: 12166504] - PubMed
CHAMPS 2003 {published data only}
-
- O'Connor P. The effects of intramuscular interferon beta‐1a in patients at high risk for development of multiple sclerosis: a post hoc analysis of data from CHAMPS. Clinical Therapeutics 2003;25(11):2865‐74. [PUBMED: 14693310] - PubMed
CHAMPS 2009 {published data only}
-
- O'Connor P, Kinkel RP, Kremenchutzky M. Efficacy of intramuscular interferon beta‐1a in patients with clinically isolated syndrome: analysis of subgroups based on new risk criteria. Multiple sclerosis 2009;15(6):728‐34. [PUBMED: 19482863] - PubMed
Curkendall 2011 {published data only}
-
- Curkendall SM, Wang C, Johnson BH, Cao Z, Preblick R, Torres AM, et al. Potential health care cost savings associated with early treatment of multiple sclerosis using disease‐modifying therapy. Clinical Therapeutics 2011;33(7):914‐25. [PUBMED: 21684600] - PubMed
ETOMS 2003 {published data only}
-
- Barkhof F, Rocca M, Francis G, Waesberghe JH, Uitdehaag BM, Hommes OR, et al. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon beta1a. Annals of Neurology 2003;53(6):718‐24. [PUBMED: 12783417] - PubMed
Filippi 2004 {published data only}
-
- Filippi M, Rovaris M, Inglese M, Barkhof F, Stefano N, Smith S, et al. Interferon beta‐1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double‐blind, placebo‐controlled trial. Lancet 2004;364(9444):1489‐96. [PUBMED: 15500893] - PubMed
Kuhle 2015 {published data only}
-
- Kuhle J, Disanto G, Dobson R, Adiutori R, Bianchi L, Topping J, et al. Conversion from clinically isolated syndrome to multiple sclerosis: a large multicentre study. Multiple Sclerosis 2015;21(8):1013‐24. [PUBMED: 25680984] - PubMed
Lazzaro 2009 {published data only}
-
- Lazzaro C, Bianchi C, Peracino L, Zacchetti P, Uccelli A. Economic evaluation of treating clinically isolated syndrome and subsequent multiple sclerosis with interferon beta‐1b. Neurological Sciences 2009;30(1):21‐31. [PUBMED: 19169625] - PubMed
Meyniel 2012 {published data only}
Moraal 2009 {published data only}
-
- Moraal B, Pohl C, Uitdehaag BM, Polman CH, Edan G, Freedman MS, et al. Magnetic resonance imaging predictors of conversion to multiple sclerosis in the BENEFIT study. Archives of Neurology 2009;66(11):1345‐52. [PUBMED: 19901165] - PubMed
Mowry 2009 {published data only}
MSBASIS 2015 {published data only}
REFLEX 2014a {published data only}
-
- Freedman MS, Stefano N, Barkhof F, Polman CH, Comi G, Uitdehaag BM, et al. Patient subgroup analyses of the treatment effect of subcutaneous interferon β‐1a on development of multiple sclerosis in the randomized controlled REFLEX study. Journal of Neurology 2014;261(3):490‐9. [PUBMED: 24413638] - PMC - PubMed
REFLEX 2014b {published data only}
-
- Stefano N, Comi G, Kappos L, Freedman MS, Polman CH, Uitdehaag BM, et al. Efficacy of subcutaneous interferon β‐1a on MRI outcomes in a randomised controlled trial of patients with clinically isolated syndromes. Journal of Neurology Neurosurgery and Psychiatry 2914;85(6):647‐53. [PUBMED: 24292999] - PMC - PubMed
SWISS COHORT STUDY 2013 {published data only}
-
- Gobbi C, Zecca C, Linnebank M, Müller S, You X, Meier R, et al. Swiss analysis of multiple sclerosis: a multicenter, non‐interventional, retrospective cohort study of disease‐modifying therapies. European Neurology 2013;70(1‐2):35‐41. [PUBMED: 23689307] - PubMed
SWISS COHORT STUDY 2016 {published data only}
References to ongoing studies
NCT01013350 {unpublished data only}
-
- NCT01013350. Prospective observational long‐term safety registry of multiple sclerosis patients who have participated in cladribine clinical trials (PREMIERE). (first received 11 November 2009).
NCT01371071 {published and unpublished data}
-
- NCT01371071. Cohort study of clinically isolated syndrome and early multiple sclerosis (CIS‐COHORT) [Clinically isolated syndrome and newly diagnosed multiple sclerosis: diagnostic, prognostic and therapy ‐ response markers ‐ a Prospective Observational Study (Berlin CIS‐COHORT)]. clinicaltrials.gov/show/NCT01371071 (first received 8 June 2011).
Additional references
Active Biotech 2014
-
- Active Biotech. Teva and Active Biotech remain committed to the development of NERVENTRA® (laquinimod) for multiple sclerosis following the negative opinion from the EMA's CHMP. www.activebiotech.com/press‐releases‐1?pressurl=http://cws.huginonline.c.... (accessed 05 April 2017).
Aharoni 2014
-
- Aharoni R. Immunomodulation neuroprotection and remyelination. The fundamental therapeutic effects of glatiramer acetate: a critical review. Journal of Autoimmunity 2014;54:81‐92. [PUBMED: 24934599] - PubMed
Broadley 2014
-
- Broadley SA, Barnett MH, Boggild M, Brew BJ, Butzkueven H, Heard R, et al. Therapeutic approaches to disease modifying therapy for multiple sclerosis in adults: an Australian and New Zealand perspective: part 3 treatment practicalities and recommendations. MS Neurology Group of the Australian and New Zealand Association of Neurologists. Journal of Clinical Neuroscience 2014;21(11):1857‐65. [PUBMED: 24993136] - PubMed
Chun 2010
Claussen 2012
-
- Claussen MC, Korn T. Immune mechanisms of new therapeutic strategies in MS: teriflunomide. Clinical Immunology 2012;142:49‐56. [PUBMED: 21367665] - PubMed
Clerico 2008
-
- Clerico M, Faggiano F, Palace J, Rice G, Tintorè M, Durelli L. Recombinant interferon beta or glatiramer acetate for delaying conversion of the first demyelinating event to multiple sclerosis. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005278.pub3] - DOI - PubMed
Colombo 2014
Colombo 2016
EMA 2006
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Tysabri. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000... (accessed 05 April 2017).
EMA 2011
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Gilenya. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002... (accessed 05 April 2017).
EMA 2013a
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Aubagio. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002... (accessed 05 April 2017).
EMA 2013b
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Lemtrada. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003... (accessed 05 April 2017).
EMA 2014a
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Tecfidera. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002... (accessed 05 April 2017).
EMA 2014b
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Plegridy. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002... (accessed 05 April 2017).
EMA 2014c
-
- European Medicines Agency. Refusal of the marketing authorisation for Nerventra (laquinimod). www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_‐_Initi... (accessed 05 April 2017).
EMA 2015a
-
- European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis. Committee for Medicinal Products for Human Use (CHMP). EMA/CHMP/771815/2011, Rev. 2. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/... (accessed 05 April 2017).
EMA 2015b
-
- European Medicines Agency. Refusal of the marketing authorization for Movectro (cladribine): outcome of re‐examination. www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_‐_Initi... (accessed 05 April 2017).
EMA 2016
-
- EMA. Marketing authorisation for the medicinal product Zinbryta. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information... (accessed 05 April 2017).
Faria 2015
-
- Faria R, Hernandez Alava M, Manca A, Wailoo AJ. NICE DSU technical support document 17: the use of observational data to inform estimates of treatment effectiveness in technology appraisal: methods for comparative individual patient data. www.nicedsu.org.uk/TSD17%20‐%20DSU%20Observational%20data%20FINAL.pdf (accessed 05 April 2017).
FDA 2000
-
- U.S. Food, Drug Administration. Mitoxantrone (Novantrone) Product Approval Information ‐ Licensing Action 2000. www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21120.pdf_Novantrone_App... (accessed 05 April 2017).
FDA 2006
-
- U.S. Food, Drug Administration. FDA approves resumed marketing of Tysabri under a special distribution program. www.fda.gov/ohrms/dockets/ac/06/briefing/2006‐4208b1_02_01biogenbriefing... (accessed 05 April 2017).
FDA 2010
-
- U.S. Food, Drug Administration. Gilenya (Fingolimod) Product Approval Information 2010. www.accessdata.fda.gov/drugsatfda_docs/appletter/2010/022527s000ltr.pdf (accessed 05 April 2017).
FDA 2012
-
- U.S. Food, Drug Administration. Aubagio (Teriflunomide) Product Approval Information 2012. www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/202992Orig1s000ltr... (accessed 05 April 2017).
FDA 2012a
-
- U.S. Food, Drug Administration. Avonex® (interferon beta‐1a) intramuscular injection. Prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2012/103628s5191lbl.pdf (accessed 05 April 2017).
FDA 2012b
-
- U.S. Food, Drug Administration. Betaseron® (interferon beta‐1b). Prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2012/103471s5063s5067s5079s... (accessed 05 April 2017).
FDA 2013
-
- U.S. Food, Drug Administration. Copaxone® (glatiramer acetate injection) solution for subcutaneous injection. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020622s087lbl.pdf (accessed 05 April 2017).
FDA 2014a
-
- U.S. Food, Drug Administration. Alemtuzumab (Lemtrada) Product Approval Information. Licensing action 2014. www.accessdata.fda.gov/drugsatfda_docs/label/2014/103948s5139lbl.pdf (accessed 05 April 2017).
FDA 2014b
-
- U.S. Food, Drug Administration. Peginterferon beta‐1a (Plegridy) Product Approval Information. Licensing action 2014. www.accessdata.fda.gov/drugsatfda_docs/label/2014/125499lbl.pdf (accessed 05 April 2017).
FDA 2016
-
- U.S. Food, Drug Administration. Zinbryta™ (daclizumab) injection, for subcutaneous use. Prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761029s000lbl.pdf (accessed 13/04/2017).
FDA 2017
-
- U.S. Food, Drug Administration. Ocrevus™ (ocrelizumab) injection, for intravenous use. Prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/761053lbl.pdf (accessed 13/04/2017).
Filippi 2016
Fox 2004
-
- Fox E. Mechanism of action of mitoxantrone. Neurology 2004;12(12 Sppl 6):15‐8. [PUBMED: 15623664] - PubMed
Freedman 2014
-
- Freedman MS, Comi G, Stefano N, Barkhof F, Polman CH, Uitdehaag BM, et al. Moving toward earlier treatment of multiple sclerosis: findings from a decade of clinical trials and implications for clinical practice. Multiple Sclerosis and Related Disorders 2014;3(2):147‐55. [PUBMED: 25878002] - PubMed
Frischer 2009
Goodin 2002
-
- Goodin DS, Frohman EM, Garmany GP Jr, Halper J, Likosky WH, Lublin FD, et al. Disease modifying therapies in multiple sclerosis: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002;58(2):169‐78. [PUBMED: 11805241] - PubMed
GRADE Working Group 2004
Higgins 2003
Higgins 2008
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2012
Hu 2012
-
- Hu X, Miller L, Richman S, Hitchman S, Glick G, Liu S, et al. A novel PEGylated interferon beta‐1a for multiple sclerosis: safety, pharmacology, and biology. Journal of Clinical Pharmacology 2012;52(6):798‐808. [PUBMED: 21680782] - PubMed
Jansen 2013
Kappos 2011
-
- Kappos L, Calabresi PA, O'Connor P, Bar‐Or A, Barkhof F, Yin M, et al. Ocrelizumab in relapsing‐remitting multiple sclerosis: a phase 2, randomised, placebo‐controlled, multicentre trial. Lancet 2011;378(9805):1779‐87. [PUBMED: 22047971] - PubMed
Kieseier 2011
-
- Kieseier BC. The mechanism of action of interferon‐β in relapsing multiple sclerosis. CNS Drugs 2011;25(6):491‐502. [PUBMED: 21649449] - PubMed
Kurtzke 1983
-
- Kurtzke J. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33(11):1444‐52. [PUBMED: 6685237] - PubMed
Leist 2011
-
- Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clinical Neuropharmacology 2011;34(1):28‐35. [PUBMED: 21242742] - PubMed
Linker 2011
-
- Linker RA, Lee DH, Ryan S, Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134(3):678‐92. [PUBMED: 21354971] - PubMed
Lycke 2015
Millard 2011
Murphy 2010
Naismith 2010
NHS England 2014
-
- NHS England Clinical Reference Group for Neurosciences. Clinical commissioning policy: disease modifying therapies for patients with multiple sclerosis (MS). www.england.nhs.uk/wp‐content/uploads/2013/10/d04‐p‐b.pdf (accessed 13 April 2016).
Oh 2013
Pakpoor 2015
Palace 2013
-
- Palace J. Partnership and consent in MS treatment choice. Journal of the Neurological Sciences 2013;335:5‐8. [PUBMED: 24090756] - PubMed
Polman 2011
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13:227–31. [PUBMED: 6847134] - PubMed
RevMan 2017 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2017.
Salanti 2012
-
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80. [PUBMED: 26062083] - PubMed
Scalfari 2013
-
- Scalfari A, Neuhaus A, Daumer M, Deluca G, Muraro P, Ebers G. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA Neurology 2013;70(2):214‐22. [PUBMED: 23407713] - PubMed
Schmied 2003
-
- Schmied M, Duda PW, Krieger JI, Trollmo C, Hafler DA. In vitro evidence that subcutaneous administration of glatiramer acetate induces hyporesponsive T cells in patients with multiple sclerosis. Clinical Immunology 2003;106(3):163–74. [PUBMED: 12706402] - PubMed
Schmitz 2013
-
- Schmitz S, Adams R, Walsh C. Incorporating data from various trial designs into a mixed treatment comparison model. Statistics in Medicine 2013;32(17):2935‐49. [PUBMED: 23440610] - PubMed
Schunemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Scolding 2015
-
- Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease‐modifying treatments in multiple sclerosis. Practical Neurology 2015;15(4):273‐9. [PUBMED: 26101071] - PubMed
Singh 2011
Smith 2010
-
- Smith B, Carson S, Fu R, McDonagh M, Dana T, Chan BKS, et al. Drug Class Review: Disease‐modifying Drugs for Multiple Sclerosis: Final Update 1 Report. 2010 Aug. Portland (OR): Oregon Health & Science University, 2010. [PUBMED: 21348046 ] - PubMed
Stangel 1999
-
- Stangel M, Toyka K, Gold R. Mechanisms of high‐dose intravenous immunoglobulins in demyelinating diseases. Archives of Neurology 1999;56(6):661–3. [PUBMED: 10369303] - PubMed
Sterne 2016
Synnot 2014
Tiede 2003
Tramacere 2015
Varrin‐Doyer 2014
Verde 2015
-
- Verde PE, Ohmann C. Combining randomized and non‐randomized evidence in clinical research: a review of methods and applications. Research Synthesis Methods 2015;6(1):45‐62. [PUBMED: 26035469] - PubMed
White 2008
-
- White IR, Higgins JPT, Wood AM. Allowing for uncertainty due to missing data in meta‐analysis. 1. Two‐stage methods. Statistics in Medicine 2008;27(5):711‐27. [PUBMED: 17703496] - PubMed
Wilms 2010
-
- Wilms H, Sievers J, Rickert U, Rostami‐Yazdi M, Mrowietz U, Lucius R. Dimethyl fumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL‐1beta, TNF‐alpha and IL‐6 in an in‐vitro model of brain inflammation. Journal of Neuroinflammation 2010;7(30):2‐8. [PUBMED: 20482831] - PMC - PubMed
Wingerchuk 2014
-
- Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease‐modifying therapies and treatment strategies. Mayo Clinic Proceedings 2014;89(2):225‐40. [PUBMED: 24485135] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials


