Insulin resistance and exendin-4 treatment for multiple system atrophy
- PMID: 28334990
- PMCID: PMC6248513
- DOI: 10.1093/brain/awx044
Insulin resistance and exendin-4 treatment for multiple system atrophy
Abstract
See Stayte and Vissel (doi:10.1093/awx064) for a scientific commentary on this article. Multiple system atrophy is a fatal sporadic adult-onset neurodegenerative disorder with no symptomatic or disease-modifying treatment available. The cytopathological hallmark of multiple system atrophy is the accumulation of α-synuclein aggregates in oligodendrocytes, forming glial cytoplasmic inclusions. Impaired insulin/insulin-like growth factor-1 signalling (IGF-1) and insulin resistance (i.e. decreased insulin/IGF-1) have been reported in other neurodegenerative disorders such as Alzheimer's disease. Increasing evidence also suggests impaired insulin/IGF-1 signalling in multiple system atrophy, as corroborated by increased insulin and IGF-1 plasma concentrations in multiple system atrophy patients and reduced IGF-1 brain levels in a transgenic mouse model of multiple system atrophy. We here tested the hypothesis that multiple system atrophy is associated with brain insulin resistance and showed increased expression of the key downstream messenger insulin receptor substrate-1 phosphorylated at serine residue 312 in neurons and oligodendrocytes in the putamen of patients with multiple system atrophy. Furthermore, the expression of insulin receptor substrate 1 (IRS-1) phosphorylated at serine residue 312 was more apparent in inclusion bearing oligodendrocytes in the putamen. By contrast, it was not different between both groups in the temporal cortex, a less vulnerable structure compared to the putamen. These findings suggest that insulin resistance may occur in multiple system atrophy in regions where the neurodegenerative process is most severe and point to a possible relation between α-synuclein aggregates and insulin resistance. We also observed insulin resistance in the striatum of transgenic multiple system atrophy mice and further demonstrate that the glucagon-like peptide-1 analogue exendin-4, a well-tolerated and Federal Drug Agency-approved antidiabetic drug, has positive effects on insulin resistance and monomeric α-synuclein load in the striatum, as well as survival of nigral dopamine neurons. Additionally, plasma levels of exosomal neural-derived IRS-1 phosphorylated at serine residue 307 (corresponding to serine residue 312 in humans) negatively correlated with survival of nigral dopamine neurons in multiple system atrophy mice treated with exendin-4. This finding suggests the potential for developing this peripheral biomarker candidate as an objective outcome measure of target engagement for clinical trials with glucagon-like peptide-1 analogues in multiple system atrophy. In conclusion, our observation of brain insulin resistance in multiple system atrophy patients and transgenic mice together with the beneficial effects of the glucagon-like peptide-1 agonist exendin-4 in transgenic mice paves the way for translating this innovative treatment into a clinical trial.
Keywords: MSA; alpha-synuclein; movement disorders; neuroprotection.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
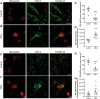
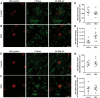
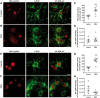
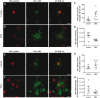


Comment in
-
New hope for devastating neurodegenerative disease.Brain. 2017 May 1;140(5):1177-1179. doi: 10.1093/brain/awx064. Brain. 2017. PMID: 29050369 Free PMC article.
Similar articles
-
Reducing C-terminal truncation mitigates synucleinopathy and neurodegeneration in a transgenic model of multiple system atrophy.Proc Natl Acad Sci U S A. 2016 Aug 23;113(34):9593-8. doi: 10.1073/pnas.1609291113. Epub 2016 Aug 1. Proc Natl Acad Sci U S A. 2016. PMID: 27482103 Free PMC article.
-
A rapidly progressive multiple system atrophy-cerebellar variant model presenting marked glial reactions with inflammation and spreading of α-synuclein oligomers and phosphorylated α-synuclein aggregates.Brain Behav Immun. 2024 Oct;121:122-141. doi: 10.1016/j.bbi.2024.07.004. Epub 2024 Jul 8. Brain Behav Immun. 2024. PMID: 38986725
-
Viral-mediated oligodendroglial alpha-synuclein expression models multiple system atrophy.Mov Disord. 2017 Aug;32(8):1230-1239. doi: 10.1002/mds.27041. Epub 2017 May 29. Mov Disord. 2017. PMID: 28556404
-
Multiple system atrophy: alpha-synuclein and neuronal degeneration.Neuropathology. 2007 Oct;27(5):484-93. doi: 10.1111/j.1440-1789.2007.00841.x. Neuropathology. 2007. PMID: 18018485 Review.
-
Animal models of multiple system atrophy.Neuroscience. 2012 Jun 1;211:77-82. doi: 10.1016/j.neuroscience.2011.09.044. Epub 2011 Sep 25. Neuroscience. 2012. PMID: 21963351 Review.
Cited by
-
Trends on Novel Targets and Nanotechnology-Based Drug Delivery System in the Treatment of Parkinson's disease: Recent Advancement in Drug Development.Curr Drug Targets. 2024;25(15):987-1011. doi: 10.2174/0113894501312703240826070530. Curr Drug Targets. 2024. PMID: 39313872 Review.
-
Pharmacotherapies for the Treatment of Progressive Supranuclear Palsy: A Narrative Review.Neurol Ther. 2024 Aug;13(4):975-1013. doi: 10.1007/s40120-024-00614-9. Epub 2024 May 14. Neurol Ther. 2024. PMID: 38743312 Free PMC article. Review.
-
The Contribution of Type 2 Diabetes to Parkinson's Disease Aetiology.Int J Mol Sci. 2024 Apr 15;25(8):4358. doi: 10.3390/ijms25084358. Int J Mol Sci. 2024. PMID: 38673943 Free PMC article. Review.
-
Sitagliptin elevates plasma and CSF incretin levels following oral administration to nonhuman primates: relevance for neurodegenerative disorders.Geroscience. 2024 Oct;46(5):4397-4414. doi: 10.1007/s11357-024-01120-4. Epub 2024 Mar 27. Geroscience. 2024. PMID: 38532069 Free PMC article.
-
Multiple system atrophy: an update and emerging directions of biomarkers and clinical trials.J Neurol. 2024 May;271(5):2324-2344. doi: 10.1007/s00415-024-12269-5. Epub 2024 Mar 14. J Neurol. 2024. PMID: 38483626 Free PMC article. Review.
References
-
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P. et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis 2014; 4: 337–44. - PubMed
-
- Bassil F, Fernagut PO, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification?. Prog Neurobiol 2014; 118: 1–18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous


