Reevaluating the Efficacy and Predictability of Antidepressant Treatments: A Symptom Clustering Approach
- PMID: 28241180
- PMCID: PMC5863470
- DOI: 10.1001/jamapsychiatry.2017.0025
Reevaluating the Efficacy and Predictability of Antidepressant Treatments: A Symptom Clustering Approach
Abstract
Importance: Depressive severity is typically measured according to total scores on questionnaires that include a diverse range of symptoms despite convincing evidence that depression is not a unitary construct. When evaluated according to aggregate measurements, treatment efficacy is generally modest and differences in efficacy between antidepressant therapies are small.
Objectives: To determine the efficacy of antidepressant treatments on empirically defined groups of symptoms and examine the replicability of these groups.
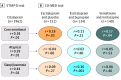
Design, setting, and participants: Patient-reported data on patients with depression from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial (n = 4039) were used to identify clusters of symptoms in a depressive symptom checklist. The findings were then replicated using the Combining Medications to Enhance Depression Outcomes (CO-MED) trial (n = 640). Mixed-effects regression analysis was then performed to determine whether observed symptom clusters have differential response trajectories using intent-to-treat data from both trials (n = 4706) along with 7 additional placebo and active-comparator phase 3 trials of duloxetine (n = 2515). Finally, outcomes for each cluster were estimated separately using machine-learning approaches. The study was conducted from October 28, 2014, to May 19, 2016.
Main outcomes and measures: Twelve items from the self-reported Quick Inventory of Depressive Symptomatology (QIDS-SR) scale and 14 items from the clinician-rated Hamilton Depression (HAM-D) rating scale. Higher scores on the measures indicate greater severity of the symptoms.
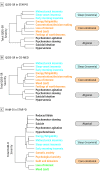
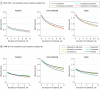
Results: Of the 4706 patients included in the first analysis, 1722 (36.6%) were male; mean (SD) age was 41.2 (13.3) years. Of the 2515 patients included in the second analysis, 855 (34.0%) were male; mean age was 42.65 (12.17) years. Three symptom clusters in the QIDS-SR scale were identified at baseline in STAR*D. This 3-cluster solution was replicated in CO-MED and was similar for the HAM-D scale. Antidepressants in general (8 of 9 treatments) were more effective for core emotional symptoms than for sleep or atypical symptoms. Differences in efficacy between drugs were often greater than the difference in efficacy between treatments and placebo. For example, high-dose duloxetine outperformed escitalopram in treating core emotional symptoms (effect size, 2.3 HAM-D points during 8 weeks, 95% CI, 1.6 to 3.1; P < .001), but escitalopram was not significantly different from placebo (effect size, 0.03 HAM-D points; 95% CI, -0.7 to 0.8; P = .94).
Conclusions and relevance: Two common checklists used to measure depressive severity can produce statistically reliable clusters of symptoms. These clusters differ in their responsiveness to treatment both within and across different antidepressant medications. Selecting the best drug for a given cluster may have a bigger benefit than that gained by use of an active compound vs a placebo.
Conflict of interest statement
Figures
Similar articles
-
New generation antidepressants for depression in children and adolescents: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 24;5(5):CD013674. doi: 10.1002/14651858.CD013674.pub2. Cochrane Database Syst Rev. 2021. PMID: 34029378 Free PMC article.
-
[Antidepressants and their onset of action: a major clinical, methodological and pronostical issue].Encephale. 2008 Jan;34(1):73-81. doi: 10.1016/j.encep.2007.12.001. Epub 2008 Feb 5. Encephale. 2008. PMID: 18514154 Review. French.
-
The impact of chronic depression on acute and long-term outcomes in a randomized trial comparing selective serotonin reuptake inhibitor monotherapy versus each of 2 different antidepressant medication combinations.J Clin Psychiatry. 2012 Jul;73(7):967-76. doi: 10.4088/JCP.11m07043. Epub 2012 May 29. J Clin Psychiatry. 2012. PMID: 22687487 Clinical Trial.
-
Irritability and Its Clinical Utility in Major Depressive Disorder: Prediction of Individual-Level Acute-Phase Outcomes Using Early Changes in Irritability and Depression Severity.Am J Psychiatry. 2019 May 1;176(5):358-366. doi: 10.1176/appi.ajp.2018.18030355. Epub 2019 Mar 29. Am J Psychiatry. 2019. PMID: 30922100
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
Cited by
-
Effects of Subchronic Buspirone Treatment on Depressive Profile in Socially Isolated Rats: Implication of Early Life Experience on 5-HT1A Receptor-Related Depression.Pharmaceuticals (Basel). 2024 Jun 1;17(6):717. doi: 10.3390/ph17060717. Pharmaceuticals (Basel). 2024. PMID: 38931384 Free PMC article.
-
Primary care physicians' perceptions of artificial intelligence systems in the care of adolescents' mental health.BMC Prim Care. 2024 Jun 13;25(1):215. doi: 10.1186/s12875-024-02417-1. BMC Prim Care. 2024. PMID: 38872128 Free PMC article.
-
Development of a harmonized sociodemographic and clinical questionnaire for mental health research: A Delphi-method-based consensus recommendation.Aust N Z J Psychiatry. 2024 Aug;58(8):656-667. doi: 10.1177/00048674241253452. Epub 2024 Jun 6. Aust N Z J Psychiatry. 2024. PMID: 38845137 Free PMC article. Review.
-
Towards precision in the diagnostic profiling of patients: leveraging symptom dynamics as a clinical characterisation dimension in the assessment of major depressive disorder.Br J Psychiatry. 2024 May;224(5):157-163. doi: 10.1192/bjp.2024.19. Br J Psychiatry. 2024. PMID: 38584324 Free PMC article.
-
Comparison of cerebrospinal fluid, plasma and neuroimaging biomarker utility in Alzheimer's disease.Brain Commun. 2024 Mar 15;6(2):fcae081. doi: 10.1093/braincomms/fcae081. eCollection 2024. Brain Commun. 2024. PMID: 38505230 Free PMC article.
References
-
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62(1):123-146. - PubMed
-
- Cipriani A, Furukawa TA, Salanti G, et al. . Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. - PubMed
-
- Rush AJ, Trivedi MH, Wisniewski SR, et al. . Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials


