CD4+ virtual memory: Antigen-inexperienced T cells reside in the naïve, regulatory, and memory T cell compartments at similar frequencies, implications for autoimmunity
- PMID: 27894837
- PMCID: PMC6066671
- DOI: 10.1016/j.jaut.2016.11.001
CD4+ virtual memory: Antigen-inexperienced T cells reside in the naïve, regulatory, and memory T cell compartments at similar frequencies, implications for autoimmunity
Abstract
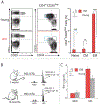
It is widely accepted that central and effector memory CD4+ T cells originate from naïve T cells after they have encountered their cognate antigen in the setting of appropriate co-stimulation. However, if this were true the diversity of T cell receptor (TCR) sequences within the naïve T cell compartment should be far greater than that of the memory T cell compartment, which is not supported by TCR sequencing data. Here we demonstrate that aged mice with far fewer naïve T cells, respond to the model antigen, hen eggwhite lysozyme (HEL), by utilizing the same TCR sequence as their younger counterparts. CD4+ T cell repertoire analysis of highly purified T cell populations from naive animals revealed that the HEL-specific clones displayed effector and central "memory" cell surface phenotypes even prior to having encountered their cognate antigen. Furthermore, HEL-inexperienced CD4+ T cells were found to reside within the naïve, regulatory, central memory, and effector memory T cell populations at similar frequencies and the majority of the CD4+ T cells within the regulatory and memory populations were unexpanded. These findings support a new paradigm for CD4+ T cell maturation in which a specific clone can undergo a differentiation process to exhibit a "memory" or regulatory phenotype without having undergone a clonal expansion event. It also demonstrates that a foreign-specific T cell is just as likely to reside within the regulatory T cell compartment as it would the naïve compartment, arguing against the specificity of the regulatory T cell compartment being skewed towards self-reactive T cell clones. Finally, we demonstrate that the same set of foreign and autoreactive CD4+ T cell clones are repetitively generated throughout adulthood. The latter observation argues against T cell-depleting strategies or autologous stem cell transplantation as therapies for autoimmunity-as the immune system has the ability to regenerate pathogenic clones.
Keywords: Autoimmunity; CD4 T cell; Driver T cells; Experimental autoimmune encephalomyelitis; Hematopoietic stem cell transplantation; Memory T cells; Next generation sequencing; T cell repertoire analysis; T regulatory cells; Virtual memory.
Published by Elsevier Ltd.
Conflict of interest statement
Figures

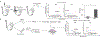
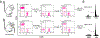

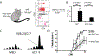

Similar articles
-
Human T-cell memory consists mainly of unexpanded clones.Immunol Lett. 2010 Sep 6;133(1):42-8. doi: 10.1016/j.imlet.2010.06.011. Epub 2010 Jul 16. Immunol Lett. 2010. PMID: 20621124
-
Depletion of CD4+ CD25+ regulatory T cells confers susceptibility to experimental autoimmune encephalomyelitis (EAE) in GM-CSF-deficient Csf2-/- mice.J Leukoc Biol. 2016 Oct;100(4):747-760. doi: 10.1189/jlb.3A0815-359R. Epub 2016 Jun 2. J Leukoc Biol. 2016. PMID: 27256565 Free PMC article.
-
Naïve subset develops the most important alloreactive response among human CD4+ T lymphocytes in human leukocyte antigen-identical related setting.Eur J Haematol. 2014 Jun;92(6):491-6. doi: 10.1111/ejh.12283. Epub 2014 Mar 15. Eur J Haematol. 2014. PMID: 24520815
-
T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire.Annu Rev Immunol. 2002;20:101-23. doi: 10.1146/annurev.immunol.20.081701.141316. Epub 2001 Oct 4. Annu Rev Immunol. 2002. PMID: 11861599 Review.
-
Antiviral memory phenotype T cells in unexposed adults.Immunol Rev. 2013 Sep;255(1):95-109. doi: 10.1111/imr.12095. Immunol Rev. 2013. PMID: 23947350 Review.
Cited by
-
HIF1α-regulated glycolysis promotes activation-induced cell death and IFN-γ induction in hypoxic T cells.Nat Commun. 2024 Oct 30;15(1):9394. doi: 10.1038/s41467-024-53593-8. Nat Commun. 2024. PMID: 39477954 Free PMC article.
-
From Genesis to Old Age: Exploring the Immune System One Cell at a Time with Flow Cytometry.Biomedicines. 2024 Jul 3;12(7):1469. doi: 10.3390/biomedicines12071469. Biomedicines. 2024. PMID: 39062042 Free PMC article. Review.
-
Oral and Extra-Oral Manifestations of Hypersensitivity Reactions in Orthodontics: A Comprehensive Review.J Funct Biomater. 2024 Jun 27;15(7):175. doi: 10.3390/jfb15070175. J Funct Biomater. 2024. PMID: 39057297 Free PMC article. Review.
-
Decoding the inflammatory signature of the major depressive episode: insights from peripheral immunophenotyping in active and remitted condition, a case-control study.Transl Psychiatry. 2024 Jun 12;14(1):254. doi: 10.1038/s41398-024-02902-2. Transl Psychiatry. 2024. PMID: 38866753 Free PMC article.
-
A randomized, placebo controlled, double blinded, parallel group clinical study to evaluate the efficacy and safety of AEV01 along with standard care for elderly patients with mild COVID-19.J Ayurveda Integr Med. 2024 Jan-Feb;15(1):100860. doi: 10.1016/j.jaim.2023.100860. Epub 2024 Feb 6. J Ayurveda Integr Med. 2024. PMID: 38320447 Free PMC article.
References
-
- Sallusto F, Lenig D, Forster R, Lipp M, & Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401(6754):708–712. - PubMed
-
- Bielekova B, et al. (2000) Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 6(10):1167–1175. - PubMed
-
- Davis MM & Bjorkman PJ (1988) T-cell antigen receptor genes and T-cell recognition. Nature 334(6181):395–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials


