Valproate for schizophrenia
- PMID: 27884042
- PMCID: PMC6734130
- DOI: 10.1002/14651858.CD004028.pub4
Valproate for schizophrenia
Abstract
Background: Many people with schizophrenia do not achieve a satisfactory treatment response with ordinary antipsychotic drug treatment. In these cases, various add-on medications are used, and valproate is one of these.
Objectives: To examine whether:1. valproate alone is an effective treatment for schizophrenia and schizoaffective psychoses; and2. valproate augmentation of antipsychotic medication is an effective treatment for the same illnesses.
Search methods: We searched the Cochrane Schizophrenia Group's Study-Based Register of Trials (July 2002; February 2007; July 2012; March 04, 2016). We also contacted pharmaceutical companies and authors of relevant studies in order to identify further trials.
Selection criteria: We included all randomised controlled trials comparing valproate to antipsychotics or to placebo (or no intervention), whether as the sole agent or as an adjunct to antipsychotic medication for the treatment of people with schizophrenia or schizophrenia-like psychoses.
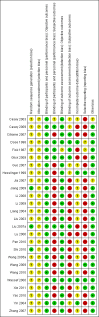
Data collection and analysis: We independently inspected citations and, where possible, abstracts, ordered papers, and re-inspected and quality-assessed these. At least two review authors independently extracted data. We analysed dichotomous data using risk ratio (RR) and its 95% confidence intervals (CI). We analysed continuous data using mean differences (MD) and their 95% CI. We assessed risk of bias for included studies and used GRADE (Grading of Recommendations Assessment, Development and Evaluation) to create a 'Summary of findings' table.
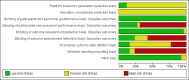
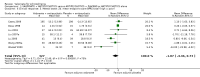
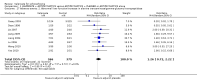
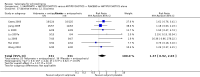
Main results: The 2012 update search identified 19 further relevant studies, most of which were from China. Thus the review currently includes 26 studies with a total of 2184 participants. All trials examined the effectiveness of valproate as an adjunct to antipsychotics. With the exception of two studies, the studies were small, the participants and personnel were not blinded (neither was outcome assessment), and most were short-term and incompletely reported.For this update we prespecified seven main outcomes of interest: clinical response (clinically significant response, aggression/agitation), leaving the study early (acceptability of treatment, overall tolerability), adverse events (sedation, weight gain) and quality of life.Adding valproate to antipsychotic treatment resulted in more clinically significant response than adding placebo to antipsychotic drugs (14 RCTs, n = 1049, RR 1.31, 95% CI 1.16 to 1.47, I2 = 12%, low-quality evidence). However, this effect was removed after excluding open RCTs in a sensitivity analysis. In terms of acceptability of treatment (measured by the number of participants leaving the study early due to any reason) valproate was just as acceptable as placebo (11 RCTs, n = 951, RR 0.76, 95% CI 0.47 to 1.24, I2 = 55%). Also overall tolerability (measured by the number of participants leaving the study early for adverse events) between valproate and placebo was similar (6 RCTs, n = 974, RR 1.33, 95% CI 0.90 to 1.97, I2 = 0).Participants in the valproate group were found to be less aggressive than the control group based on the Modified Overt Aggression Scale (3 RCTs, n = 186, MD -2.55, 95% CI -3.92 to -1.19, I2 = 82%, very low-quality evidence). Participants receiving valproate more frequently experienced sedation (8 RCTs, n = 770, RR 1.38, 95% CI 1.07 to 1.79, I2 = 0, low-quality evidence) but were no more likely to gain weight than those receiving placebo (4 RCTs, n = 427, RR 1.17, 95% CI 0.76 to 1.82, I2 = 0, low-quality evidence). No study reported on the important outcome of quality of life.
Authors' conclusions: There is limited evidence, based on a number of trials, that the augmentation of antipsychotics with valproate may be effective for overall clinical response, and also for specific symptoms, especially in terms of excitement and aggression. However, this evidence was entirely based on open RCTs. Moreover, valproate was associated with a number of adverse events among which sedation and dizziness appeared significantly more frequently than in the control groups. Further randomised studies which are blinded are necessary before any clear recommendation can be made. Ideally these would focus on people with schizophrenia and aggression, on those with treatment-resistant forms of the illness and on those with schizoaffective disorders.
Conflict of interest statement
Yijun Wang: none known.
Jun Xia: none known.
Bartosz Helfer: none known.
Chunbo Li: none known.
Stefan Leucht has received honoraria for lectures from Otsuka, Lundbeck, Janssen, ICON, Lilly, SanofiAventis, AOP Orphan and Servier; for consulting from Janssen, Roche, Lilly, Otsuka, Lundbeck, TEVA and for a publications from Roche.
Figures































































Update of
-
Valproate for schizophrenia.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD004028. doi: 10.1002/14651858.CD004028.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2016 Nov 24;11:CD004028. doi: 10.1002/14651858.CD004028.pub4. PMID: 18646098 Updated. Review.
Comment in
-
Valproate for schizophrenia: ambrosia?CNS Spectr. 2021 Aug;26(4):314-315. doi: 10.1017/S1092852920001285. Epub 2020 Apr 3. CNS Spectr. 2021. PMID: 32241333 No abstract available.
Similar articles
-
Valproate for schizophrenia.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD004028. doi: 10.1002/14651858.CD004028.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2016 Nov 24;11:CD004028. doi: 10.1002/14651858.CD004028.pub4. PMID: 18646098 Updated. Review.
-
Valproate for schizophrenia.Cochrane Database Syst Rev. 2004;(1):CD004028. doi: 10.1002/14651858.CD004028.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2008 Jul 16;(3):CD004028. doi: 10.1002/14651858.CD004028.pub3. PMID: 14974054 Updated. Review.
-
Lithium for schizophrenia.Cochrane Database Syst Rev. 2015 Oct 28;2015(10):CD003834. doi: 10.1002/14651858.CD003834.pub3. Cochrane Database Syst Rev. 2015. PMID: 26509923 Free PMC article. Review.
-
Carbamazepine for schizophrenia.Cochrane Database Syst Rev. 2014 May 2;2014(5):CD001258. doi: 10.1002/14651858.CD001258.pub3. Cochrane Database Syst Rev. 2014. PMID: 24789267 Free PMC article. Review.
-
Benzodiazepines for schizophrenia.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD006391. doi: 10.1002/14651858.CD006391. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Nov 14;11:CD006391. doi: 10.1002/14651858.CD006391.pub2. PMID: 17253592 Updated. Review.
Cited by
-
How pharmacology can aid in the diagnosis of mental disorders.Naunyn Schmiedebergs Arch Pharmacol. 2024 Sep 4. doi: 10.1007/s00210-024-03413-z. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39230588 Review.
-
Orlistat for the treatment of antipsychotic-induced weight gain: an eight-week multicenter, randomized, placebo-controlled, double-blind trial.Lipids Health Dis. 2024 Jul 24;23(1):225. doi: 10.1186/s12944-024-02214-w. Lipids Health Dis. 2024. PMID: 39049073 Free PMC article. Clinical Trial.
-
Treatment of Aggressive Behavior and Agitation in an 11-Year-Old Boy with Co-Occurring Autism and ADHD: A Case Report and Literature Review on the Use of Intravenous Valproate in Emergency Psychiatry.J Clin Med. 2024 Jun 18;13(12):3573. doi: 10.3390/jcm13123573. J Clin Med. 2024. PMID: 38930101 Free PMC article. Review.
-
Permissive epigenetic regulatory mechanisms at the histone level are enhanced in postmortem dorsolateral prefrontal cortex of individuals with schizophrenia.J Psychiatry Neurosci. 2024 Feb 1;49(1):E35-E44. doi: 10.1503/jpn.230054. Print 2024 Jan-Feb. J Psychiatry Neurosci. 2024. PMID: 38302137 Free PMC article.
-
Global Neuropsychopharmacological Prescription Trends in Adults with Schizophrenia, Clinical Correlates and Implications for Practice: A Scoping Review.Brain Sci. 2023 Dec 20;14(1):6. doi: 10.3390/brainsci14010006. Brain Sci. 2023. PMID: 38275511 Free PMC article. Review.
References
References to studies included in this review
Casey 2003 {published data only}
-
- Casey DE, Daniel DG, Wassef AA, Tracy KA, Wolnlak P, Sommerville W. Effect of divalproex combined with olanzapine or risperidone in participants with acute exacerbation of schizophrenia. Neuropsychopharmacology 2003;28:182‐92. - PubMed
-
- Citrome L, Casey DE, Daniel DG, Wozniak P, Kochan LD, Tracy KA. Adjunctive divalproex and hostility among participants with schizophrenia receiving olanzapine or risperidone. Psychiatric Services 2004;55(3):290‐4. [MEDLINE: ; Embase 2004111171] - PubMed
-
- Jafari M, Jiang P, Casey DE. Adjunctive divalproex sodium lowers cholesterol elevation with olanzapine. 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York, New York, USA. 2004. [APA Conference Abstracts NR634]
Casey 2009 {published data only}
-
- Casey DE, Daniel DG, Tamminga C, Kane JM, Tran‐Johnson T, Wozniak P, et al. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacology 2009;34(5):1330‐8. [MEDLINE: ] - PubMed
-
- M02‐547. A randomized, double‐blind study of the safety and efficacy of Depakote ER plus an atypical antipsychotic vs. an atypical antipsychotic alone in the treatment of schizophrenia. www.abbvie.com/content/dam/abbviecorp/us/desktop/research/clinical‐trial... [accessed 08 April 2016].
-
- NCT00073164. A study of the safety and efficacy of Depakote ER plus an atypical antipsychotic vs an atypical antipsychotic alone in the treatment of schizophrenia. clinicaltrials.gov/ct2/show/NCT00073164 [Date Accessed: 17 November 2003].
Citrome 2007 {published data only}
-
- Citrome L. Schizophrenia and valproate. Psychopharmacology Bulletin. United States of America, 2003; Vol. 37, issue Suppl 2:74‐88. - PubMed
-
- Citrome L, Daniel D, Wassef A, Tracy K, Wozniak P, Casey D. Antipsychotic monotherapy versus combination treatment with valproate in hospitalized patients with acute schizophrenia: a double‐blind, multi‐center study. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA 2002:NR316.
-
- Citrome L, Shope CB, Nolan KA, Czobor P, Volavka J. Risperidone alone versus risperidone plus valproate in the treatment of participants with schizophrenia and hostility. International Clinical Psychopharmacology 2007;22(6):356‐62. [MEDLINE: ] - PubMed
-
- NCT00308360. Risperidone alone vs risperidone plus valproate in the treatment of participants with schizophrenia and hostility. clinicaltrials.gov/ct2/show/NCT00308360 [Date Accessed: 28 March 2006].
-
- Nolan K, Shope C, Citrome L, Volavka J. Staff and patient views of the reasons for aggressive incidents: a prospective, incident‐based study. Psychiatric Quarterly. United States, 2009; Vol. 80, issue 3:167‐72. - PubMed
Dose 1998 {published data only}
-
- Dose M. Die Bedeutung von Antikonvulsiva und Calciumantagonisten für die Pharmakotherapie von Psychosen. Habilitationsschrift. Technische Universität München, 1991.
-
- Dose M, Hellweg R, Yassouridis A, Theison M, Emrich HM. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry 1998;31(4):122‐5. [MEDLINE: ] - PubMed
Fisk 1987 {published data only}
-
- Fisk GG, York SM. The effect of sodium valproate on tardive dyskinesia ‐ revisited. British Journal of Psychiatry 1987;150:542‐6. [MEDLINE: ] - PubMed
Glick 2009 {published data only}
-
- Glick ID, Bosch J, Casey DE. A double‐blind randomized trial of mood stabilizer augmentation using lamotrigine and valproate for participants with schizophrenia who are stabilized and partially responsive. Journal of Clinical Psychopharmacology 2009;29(3):267‐71. [MEDLINE: ] - PubMed
Guo 2007 {published data only}
-
- Guo CR. Study of quetiapine combined with magnesium valproate release tablets in treatment of schizophrenia with symptoms of elation and agitation [奎硫平合并丙戊酸镁缓释片治疗精神分裂症兴奋激越研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007;17(3):183‐4.
Hesslinger 1999 {published data only}
-
- Hesslinger B, Normann C, Langosch JM, Klose P, Berger M, Walden J. Effects of carbamazepine and valproate on haloperidol plasma levels and on psychopathologic outcome in schizophrenic participants. Journal of Clinical Psychopharmacology 1999;19(4):310‐5. [MEDLINE: ] - PubMed
-
- Normann C, Klose P, Hesslinger B, Langosch J, Berger M, Walden J. Haloperidol plasma levels and psychopathology in schizophrenic patients with antiepileptic co‐medication: a clinical trial. PharmacoPsychiatry 1997; Vol. 30:204.
-
- Walden J, Hesslinger B, Normann C, Langosch J, Berger M. Actions of carbamazepine and valproate on haloperidol plasma levels and psychopathological outcome. Proceedings of the 21st Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1998 Jul 12‐16; Glasgow, UK 1998.
Jia 2007 {published data only}
-
- Jia HX, Bian ZY, Zhu H, Jiang T, Wang LF, Liu HB, et al. Effect of sodium valproate on serum concentration and clinical efficacy of clozapine [丙戊酸钠对氯氮平血药浓度和疗效的影响]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007;17(5):310‐1.
Jiang 2009 {published data only}
-
- Jiang CY, Chen JX, He GQ. The effect of adjuvant magnesium valproate sustained ‐ release tablets for schizophrenia with agitation and agitation. [丙戊酸镁缓释片辅助治疗精神分裂症兴奋激越的效果观察]. Chinese Magazine of Drug Abuse Prevention and Treatment [中国药物滥用防治杂志] 2009;15(3):175‐7.
Li 2008 {published data only}
-
- Li WS, Yu XD, Cui Y. A comparative study of olanzapine combined with low‐dose magnesium valproate sustained‐release tablets in the treatment of acute phrase of schizophrenia [奥氮平合并小剂量丙戊酸镁缓释片治疗急性期精神分裂症的对照研究]. Journal of Psychiatry [精神医学杂志] 2008;21(3):178‐9.
Li 2009 {published data only}
-
- Li SS. The effect of aripiprazole combined with magnesium valproate in the treatment of impulse control disorder of schizophrenia [阿立哌唑联合丙戊酸镁对精神分裂症冲动控制障碍的疗效观察]. Chongqing Medical Journal [重庆医学杂志] 2009;38(7):788, 798.
Liang 2004 {published data only}
-
- Liang HX, Du JY, Wu ZJ. A study of additive effect of sodium‐valproate in the treatment of schizophrenia. Journal of Clinical Psychological Medicine 2004;14(3):151‐2.
-
- Ma XW. The effect of adjuvant magnesium valproate on schizophrenia [丙戊酸镁对精神分裂症的辅助治疗作用]. China Practical Medicine [中国实用医药] 2010;5(12):43﹣4.
Liu 2003 {published data only}
-
- Liu C. The effect of sodium valproate in participants with schizophrenia. Chinese Journal of Clinical Medicinal Professional Research 2003;70:11, 596‐7.
Liu 2007a {published data only}
-
- Liu Z. Sodium valproate in treatment of aggression for psychiatric participants [丙戊酸钠治疗精神病患者的攻击行为]. Chongqing Journal of Medicine [重庆医学杂志] 2007;36(6):490‐1.
Lu 2006 {published data only}
-
- Lu W, Jin W, Wang H, Ma Y, Tong Z. Comparing study of sodium valproate xr (Depakine) in the supplementary therapy of excitement state in participants with schizophrenia and schizoaffective disorder. Proceedings of the Zhejiang Annual Psychiatry Conference, Yiwu, China. 2005:75‐7.
-
- Lu WM, Jin WD, Shen Y, Ma YC, Tong ZH. Clinical evaluation of sodium valproate xr (Depakine) in adjunctive therapy for participants with excitomania associated with schizophrenia and schizoaffective disorder. Chinese New Drugs Journal 2006;15(15):1291‐3.
Pan 2010 {published data only}
-
- Pan ZX, Yin DF, Liu NH. Sodium valproate combined clozapine in the treatment of refractory schizophrenia 34 cases [丙戊酸钠联合氯氮平治疗难治性精神分裂症34例]. Herald of Medicine [医学导报] 2010;29(2):203‐4.
Shi 2010 {published data only}
-
- Shi YX, Dai TG, Yi PC. A study of olanzapine combined with magnesium valproate sustained‐release tablets for the treatment of excitement and agitation in acute phase of schizophrenia [奥氮平合并丙戊酸镁缓释片治疗精神分裂症急性期兴奋激越研究]. Modern Practical Medicine [现代实用医学] 2010;22(4):398‐9.
Wang 2005a {published data only}
-
- Wang H. Combination of magnesium valproate and anti‐psychotics in aggressive behaviors of schizophrenics. Journal of Clinical Psychosomatic Diseases 2005;11(1):10‐1. [113903]
Wang 2009 {published data only}
-
- Wang GP, Xie R, Pei GX, Zhang XJ. The effect of adjuvant magnesium valproate sustained‐release tablets in the treatment of schizophrenia [丙戊酸镁缓释片治疗精神分裂症的辅助作用]. Journal of Clinical Psychiatry [临床精神医学杂志] 2009;19(4):250‐1.
Wang 2010 {published data only}
-
- Wang LJ, Zhang J. A comparative study of sodium valproate combined with other drugs for schizophrenia with impulsive and aggressive behavior [合用丙戊酸钠治疗伴有冲动和攻击行为的精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2010;22(5):548‐9.
Wassef 2000 {published data only}
-
- Wassef AA, Dott SG, Harris A, Brown A, O'Boyle M, Meyer WJ 3rd, et al. Randomized, placebo‐controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. Journal of Clinical Psychopharmacology 2000;20(3):357‐61. [MEDLINE: ] - PubMed
Xie 2011 {published data only}
-
- Xie R, Wang G, Pei G. The effect of adjuvant magnesium valproate sustained‐release tablet in the treatment of schizophrenic participants with aggressive behaviours [丙戊酸镁缓释片辅助治疗精神分裂症攻击行为的疗效观察]. Journal of Clinical Psychiatry [临床精神医学杂志] 2011;21(3):191‐2.
Yao 2010 {published data only}
-
- Yao F, Jiang F. Short‐term efficacy of risperidone combined with magnesium valproate sustained‐release tablets in the treatment of schizophrenia with aggressive behavior [利培酮联合丙戊酸镁缓释片治疗精神分裂症攻击行为的近期疗效]. Occupation and Health [职业与健康] 2010;26(22):2713‐4.
Yin 2004 {published data only}
-
- Yin XR, Xie BQ, Jiang L. A double‐blind comparative study of sodium valproate in treating of TD. Journal of Clinical Psychological Medicine 2004;14(2):92‐3. [113903]
Zhang 2007 {published data only}
-
- Zhang SL, Wang CX, Wang LT, Feng YF. The effect of debakin combined with risperidone in the treatment of schizophrenia with psychmotor excitement [德巴金联合利培酮治疗伴精神运动性兴奋的精神分裂症的效果]. Medical Journal of Qilu [齐鲁医学杂志] 2007;22(3):265‐6.
References to studies excluded from this review
Alberti 1999 {published data only}
-
- Alberti GG, Garini R, Marzolini M, Beltz J, Greco MI, Maresca G. Efficacy and tolerability of gabapentin (GBP) and valproate (VPA) as an adjunct in the neuroleptic treatment of acute manic syndromes. Journal of the European College of Neuropsychopharmacology 1999;9:210.
Bersudsky 2010 {published data only}
-
- Bersudsky Y, Applebaum J, Gaiduk Y, Sharony L, Mishory A, Podberezsky A, et al. Valnoctamide as a valproate substitute with low teratogenic potential in mania: a double‐blind, controlled, add‐on clinical trial. Bipolar Disorders 2010;12(4):376‐82. - PubMed
Centorrino 1994 {published data only}
-
- Centorrino F, Baldessarini RJ, Kando J, Frankenburg FR, Volpicelli SA, Puopolo PR, et al. Serum concentrations of clozapine and its major metabolites: effects of co‐treatment with fluoxetine or valproate. American Journal of Psychiatry 1994;151(1):123‐5. [MEDLINE: ] - PubMed
Haupt 2007 {published data only}
-
- Haupt DW, Fahnestock PA, Hessler MJ, Flavin K, Bagley S, Yingling M, et al. Changes in direct measures of adiposity in schizophrenia participants during divalproex augmentation of antipsychotic treatment. Schizophrenia Bulletin 2007;33(2):501.
Ma 2010 {published data only}
-
- Ma XW. The effect of adjuvant magnesium valproate in the treatment of schizophrenia [丙戊酸镁对精神分裂症的辅助治疗作用]. China Practical Medicine [中国实用医药] April 2010;5(12):43‐4.
Monfort 1991 {published data only}
-
- Monfort JC, Mager C, Thorpe D, Ropert R, Ferrey G, Mentre F, et al. Valproate‐diazepam combination versus chlorpromazine: a double‐blind study in schizophrenic and schizophreniform disorders [Valproate‐diazépam versus chlorpromazine: étude en double aveugle dans les troubles schizophréniques et schizophréniformes]. Encephale 1991;17(2):101. [MEDLINE: ]
NCT00183443 {published data only}
-
- NCT00183443. Divalproex extended release and placebo, lithium, or quetiapine for mania. clinicaltrials.gov/ct2/show/NCT00183443 [Date Accessed: 13 September 2005].
Ping 1994 {published data only}
-
- Ping Y, Xinjuan OY, Lin T. A controlled study of sodium valproate and chlorpromazine for schizophrenia. Shanghai Archives of Psychiatry 1994;6(4):208‐9.
Raja 2000 {published data only}
-
- Raja M, Azzoni A. Second generation antipsychotics in the emergency care setting. A prospective naturalistic study. General Hospital Psychiatry 2000;22(2):107‐14. [MEDLINE: ] - PubMed
Rifang 2001 {published data only}
-
- Rifang C, Fenfang Z. The impact of sodium valproate for social function rehabilitation in schizophrenia participants. Modern Rehabilitation 2001;5(9):100.
Zhu 2005 {published data only}
-
- Zhu C, Fu R, Yang Y. Sodium valproate and clonazepam for the treatment of agitated symptoms of participants with schizophrenia: a controlled trial. Medical Journal of the Chinese People's Armed Police Forces 2005;16(12):934‐5. [CAJ MEDI0602]
References to studies awaiting assessment
Boylan 2004 {published data only}
-
- Boylan L, Labovitz D. Unbalanced statistical analysis of combined divalproex and antipsychotic therapy for schizophrenia. Neuropyschopharmacology. United States of America, 2004; Vol. 29, issue 3:636. - PubMed
Chien 1978 {published data only}
-
- Chien C, Jung K, Ross‐Townsend A. Efficacies of agents related to GABA, dopamine, and acetylcholine in the treatment of tardive dyskinesia. Psychopharmacology Bulletin 1978; Vol. 14, issue 2:20‐2. - PubMed
Cui 2012 {published data only}
-
- Cui LJ, Shen WM. Effects of magnesium valproate treatment on weight and blood insulin levels in patients with schizophrenia [丙戊酸镁对精神分裂症患者体重及胰岛素水平的影响]. Frontiers of Medicine [医药前沿] 2012; Vol. 22:97.
Ellenor 1995 {published data only}
-
- Ellenor G, Lohr J, Ito M, Dishman B, Gammariello A. The comparative efficacy of divalproex low and high serum level in bipolar disorder and schizophrenia. Psychopharmacology Bulletin 1995; Vol. 31, issue 3:563.
Friis 1983 {published data only}
-
- Friis T, Christensen TR, Gerlach J. Sodium valproate and biperiden in neuroleptic‐induced akathisia, parkinsonism and hyperkinesia. A double‐blind cross‐over study with placebo. Acta Psychiatrica Scandinavica 1983;67(3):178‐87. [MEDLINE: ] - PubMed
Gong 2010 {published data only}
-
- Gong Y, Guo P. Effects of magnesium valproate on cognitive function in patients with refractory schizophrenia [丙戊酸镁对难治性精神分裂症患者认知功能的影响]. Chronic Pathematology Journal [慢性病学杂志] 2010; Vol. 12, issue 2:107‐9.
Gu 2012 {published data only}
-
- Gu P, Wang J, Cao J, Li B‐z. A comparative study of quetiapine with sustained‐release tablets of magnesium valproate in treatment of old patients with acute phase of schizophrenia [奎硫平合并丙戊酸镁治疗老年精神分裂症急性期的对照研究]. Chinese Journal of Clinical Psychology [中国临床心理学杂志] 2012; Vol. 20, issue 4:520‐2.
Gu 2014 {published data only}
-
- Gu Q. Prognostic analysis of magnesium valproate combined with antipsychotics for patients with convalescent schizophrenia [丙戊酸镁联合抗精神病药物治疗对恢复期精神分裂症患者的预后分析]. Medical Journal of Chinese People's Health [中国民康医学] 2014; Vol. 26, issue 18:59, 99.
Huang 2013 {published data only}
-
- Huang WD, Luo HF, Lan L, Li CH, Xu LH, Li ZM. Efficacy of ziprasidone combined with low‐dose sodium valproate in the treatment of female refractory schizophrenia [齐拉西酮联合小剂量丙戊酸钠治疗女性难治性精神分裂症疗效分析]. Youjiang Medical Journal [右江医学] 2013, issue 02:159‐62.
Jiang 2014 {published data only}
-
- Jiang TC, Xiong Y. Efficacy of quetiapine combined with sodium valproate in the treatment of schizophrenia with impulsive and aggression in female patients [喹硫平联合丙戊酸钠治疗女性精神分裂症患者冲动攻击行为的疗效观察]. World Latest Medicine Information [世界最新医学信息文摘(连续型电子期刊)] 2014; Vol. 14, issue 32:79‐80.
Jin 2013 {published data only}
-
- Jin G. Efficacy of magnesium valproate for schizophrenia with impulsive and aggressive behavior [丙戊酸镁对精神分裂症冲动攻击行为疗效观察]. Chinese Journal of Modern Drug Application [中国现代药物应用] 2013, issue 14:160‐1.
Lee 1996 {published data only}
-
- Lee MS, Choi BH, Kim SH. Combined use of carbamazepine and valproic acid in negative symptom schizophrenia. 9th Congress of the European College of Neuropsychopharmacology; 1996 Sep 21‐25; Amsterdam, The Netherlands. 1996. [MEDLINE: ]
Li 2008a {published data only}
-
- Li W, Yu X, Cui Y. A comparative study of olanzapine with small‐dose sustained‐release tablets of magnesium valproate in treatment of acute phase of schizophrenia. Shandong Archives of Psychiatry 2008;21(3):178‐9.
Li 2012a {published data only}
-
- Li DM. Clinical efficacy of ziprasidone combined with valproate in the treatment of refractory schizophrenia [齐拉西酮联合丙戊酸钠治疗难治性精神分裂症的临床疗效观察]. China Medical Engineering [中国医学工程] 2012, issue 01:92‐3.
Li 2013 {published data only}
-
- Li C, Liu Y, Wang M. The effect of adjuvant magnesium valproate sustained‐release tablets combined with risperidone in the treatment of schizophrenia with impulsive aggression [丙戊酸镁缓释片合用利培酮对精神分裂症冲动攻击行为的辅助治疗]. Journal of Practical Medical Techniques [实用医技杂志] 2013, issue 01:69‐71.
Li 2013a {published data only}
-
- Li J, Chen LS, Chen Wang Y. A comparative study of effect of sodium valproate on cognitive dysfunction in patients with schizophrenia [丙戊酸钠改善精神分裂症患者认知功能障碍的对照研究]. Journal of Psychiatry [精神医学杂志] 2013, issue 04:292‐4.
Li 2014 {published data only}
-
- Li YM, Sun WB, Yang CQ. A study of low‐dose quetiapine combined with magnesium valproate in the treatment of schizophrenia [低剂量喹硫平合并丙戊酸镁治疗精神分裂症攻击行为疗效研究]. Medical Information [医学信息] 2014; Vol. 27, issue 24:47‐8.
Li 2014a {published data only}
-
- Li Y. A comparative study of antipsychotic drugs combined with low‐dose sodium valproate in the treatment of schizophrenia [抗精神病药物联合小剂量丙戊酸镁治疗精神分裂症攻击行为的对照研究]. The Chinese and Foreign Health Abstract [中外健康文摘] 2014, issue 11:53.
Liang 2004a {published data only}
-
- Liang H‐X, Du J‐Y, Wu Z‐J. A study of additive effect of sodium‐valproate in the treatment of schizophrenia. Journal of Clinical Psychological Medicine 2004;14(2):92‐3.
Linnoila 1976a {published data only}
-
- Linnoila M, Viukari M, Kietala O. Effect of sodium valproate on tardive dyskinesia. British Journal of Psychiatry 1976;129:114‐9. [MEDLINE: ] - PubMed
Linnoila 1976b {published data only}
-
- Linnoila M, Viukari M, Hietala O. Sodium valproate therapy for tardive dyskinesia. International Drug Therapy Newsletter 1976;11(Nov):33‐5. [MEDLINE: ]
Liu 2007b {published data only}
-
- Liu Z. Sodium valproate in treatment of aggression of psychiatric participants [丙戊酸钠治疗精神病患者的攻击行为]. Chongqing Medical Journal [重庆医学] 2007;36(6):490‐1.
Liu 2012 {published data only}
-
- Liu Z, Wang L, Sun Y. Clinical observation of magnesium valproate sustained‐release tablets for schizophrenia with excitement [丙戊酸镁缓释片对精神分裂症兴奋状态的疗效观察]. China Journal of Health Psychology [中国健康心理学杂志] 2012; Vol. 20, issue 11:1630‐1.
Liu 2012a {published data only}
-
- Liu J. The effect of adjuvant magnesium valproate on schizophrenia with aggressive behavior [丙戊酸镁对精神分裂症兴奋激越行为的辅助疗效观察]. Journal of Community Medicine [社区医学杂志] 2012; Vol. 10, issue 14:31‐2.
Liu 2013 {published data only}
-
- Liu Z, Song S, Wang L. Effects of magnesium valproate sustained‐release tablets combined with risperdal on lipid metabolism in patients with schizophrenia [丙戍酸镁缓释片合并维思通对精神分裂症患者脂代谢影响]. China Journal of Health Psychology [中国健康心理学杂志] 2013; Vol. 21, issue 11:1631‐2.
Liu 2014 {published data only}
-
- Liu Z, Li HJ, Pei SY. Effects of risperidone and magnesium valproate on blood concentration in patients with schizophrenia [丙戍酸镁合并利培酮治疗精神分裂症血药浓度变化及临床疗效]. China Journal of Health Psychology [中国健康心理学杂志] 2014; Vol. 22, issue 8:1141‐3.
Lv 2014 {published data only}
-
- Lv S. Effects of magnesium valproate sustained‐release tablets on cognitive function in first‐episode schizophrenic patients [丙戊酸镁缓释片对首发精神分裂症病人认知功能的影响]. China & Foreign Medical Treatment [中外医疗] 2014, issue 17:120‐1.
Ma 2012 {published data only}
-
- Ma TB. Analysis of effects of valproic acid sodium jointed with ziprasidone in the treatment of refractory schizophrenia [丙戊酸钠联合齐拉西酮治疗难治性精神分裂症的效果分析]. China Modern Medicine [中国当代医药] 2012, issue 04:73‐4.
Monteleone 1986 {published data only}
-
- Monteleone P, Maj M, Iovino M, Steardo L. Growth hormone response to sodium valproate in chronic schizophrenia. Biological Psychiatry 1986; Vol. 21, issue 7:588‐94. - PubMed
Monteleone 1988 {published data only}
-
- Monteleone P, Maj M, Ariano M, Iovino M, Fiorenza L, Steardo L. Prolactin response to sodium valproate in schizophrenics with and without tardive dyskinesia. Psychopharmacology 1988; Vol. 96, issue 2:223‐6. - PubMed
Nair 1980 {published data only}
-
- Nair N, Lal S, Schwartz G. Effect of sodium valproate and baclofen in tardive dyskinesia: clinical and neuroendocrine studies. In: Cattabeni FRG editor(s). Long Term Effects of Neuroleptics. Advances in Biochemistry and Psychopharmacology. Vol. 24, New York, USA: Raven Press, 1980:437‐41. - PubMed
Nasrallah 1986 {published data only}
-
- Nasrallah H, Dunner F, McCalley Whitters M, Smith R. Pharmacologic probes of neurotransmitter systems in tardive dyskinesia: implications for clinical management. Journal of Clinical Psychological Medicine. United‐States, 1986; Vol. 47, issue 2:56‐9. - PubMed
NCT00552500 {published data only}
-
- NCT00552500. Effects of atypical antipsychotic and valproate combination therapy on glucose and lipid metabolism in schizophrenia. clinicaltrials.gov/ct2/show/NCT00552500 [Date Accessed: 1 November 2007].
Peng 2012 {published data only}
-
- Peng ZY, Xia CL, Liu XM. A comparative study of clozapine and clozapine unite magnesium valproate in the treatment of patients with treatment‐resistant schizophrenia [氯氮平合并丙戊酸镁治疗难治性精神分裂症的对照研究]. World Health Digest Medical Periodieal [中外健康文摘] 2012; Vol. 38:164‐6.
Peng 2013 {published data only}
-
- Peng H. Effect of magnesium valproate on aggressive behavior in schizophrenia [丙戊酸镁对精神分裂症攻击行为的疗效观察]. Chinese Journal of Trauma and Disability Medicine [中国伤残医学] 2013, issue 07:187‐8.
Pu 2013 {published data only}
-
- Pu QX, Wu HB, Huang X, Yu GH, Wang DF, Gu ZW, et al. Contrast analysis of olanzapine combined with sodium valproate on refractory schizophrenia [奥氮平合并丙戊酸钠治疗难治性精神分裂症的对照分析]. Progress in Modern Biomedicine [现代生物医学进展] 2013, issue 18:3503‐6.
Qiu 2014 {published data only}
-
- Qiu LL, Wang JF. Effect of sodium valproate on risperidone therapy and blood concentration [丙戊酸镁对利培酮疗效及血药浓度的影响]. Medical Journal of Liaoning [辽宁医学杂志] 2014; Vol. 28, issue 1:9‐10.
Qu 2014 {published data only}
-
- Qu CH, Liu XL, Sun XH. A comparative study of valproate combined with olanzapine in the treatment of schizophrenia [丙戊酸钠合用奥氮平治疗精神分裂症患者攻击行为的对照研究]. Medical Information [医学信息] 2014; Vol. 27, issue 21:602.
Ruiz‐Doblado 2010 {published data only}
-
- Ruiz‐Doblado S, Baena‐Baldomero A, Esparrago‐Llorca G. Pharmacological augmentation strategies in clozapine‐resistant schizophrenia: overcoming the resistance. Psiquiatria Biologica 2010;17(3):96‐101.
Shu 2014 {published data only}
-
- Shu Z, Zhou W. Clinical observation of clozapine combined with magnesium valproate sustained‐release tablets in the treatment of schizophrenia [氯氮平联合丙戊酸镁缓释片治疗精神分裂症攻击行为的疗效观察]. Guide of China Medicine [中国医药指南] 2014; Vol. 12, issue 2:28‐9.
Su 2013 {published data only}
-
- Su L, Xu Y, Shi S, Dutt A, Bramon E. Effects of chlorpromazine, clozapine and valproate on auditory P300 in schizophrenia: a prospective longitudinal randomized controlled study. Schizophrenia Bulletin 2013; Vol. 39:S51.
Sun 2007 {published data only}
-
- Sun Q, Quo H, Zhang H, Liu Y, Zhu Y, Zhang A. The effects of magnesium valproate sustained release tablets on the cognitive function in treatment‐resistant schizophrenic participants. Proceedings of the Shanghai Regional Meeting and CSP Annual Congress; 2007 Sep 20‐23; Shanghai. 2007.
Sun 2008 {published data only}
-
- Sun Q‐X, Guo H, Liu Y. Effect of magnesium valproate sustained release tablets on cognition function in difficult cure sex schizophrenic. Chinese Journal of Modern Drug Application [中国现代药物应用] 2008;2(22):25‐7.
Sun 2012 {published data only}
-
- Sun XG. A comparative study of quetiapine combined with haloperidol, sodium valproate and clozapine in the treatment of refractory schizophrenia [奎硫平合并氟哌啶醇、丙戊酸钠与氯氮平治疗难治性分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2012; Vol. 24, issue 13:1566‐7.
Suzana 2009 {published data only}
-
- Suzana S, Olivera Z, Gordana N, Violeta S, Dragoslava G, Vladica S. Adjunctive mood stabilising treatment: the effects on hostility and impulsivity among patients with schizophrenia. European Neuropsychopharmacology 2009; Vol. 19:S532.
Tang 2012 {published data only}
-
- Tang SY, Qin CL, Zhang W, Xiong MQ. Clinical observation of sodium valproate sustained‐release tablets on schizophrenia hostile attack [丙戊酸钠治疗精神分裂症敌意攻击行为疗效观察]. J Medical Forum [医药论坛杂志] 2012; Vol. 9:36‐7.
Wang 2005b {published data only}
-
- Wang H. Combination of magnesium valproate and anti‐psychotics in aggressive behaviors of schizophrenics. Journal of Clinical Psychosomatic Diseases 2005;11(1):10‐1.
Wang 2006 {published data only}
-
- Wang HL. Therapeutic effect of risperidone combined with magnesium valproate in aggressive behaviors of schizophrenics. Chinese Journal of Health Psychology 2006;14(4):441‐2.
Wang 2012 {published data only}
-
- Wang G, Xie R, Pei G. A comparative study of quetiapine combined with magnesium valproate sustained‐release tablets in the treatment of schizophrenia with aggressive behavior [喹硫平合并丙戊酸镁缓释片治疗精神分裂症攻击行为的对照研究]. Journal of Psychiatry [精神医学杂志] 2012, issue 01:59‐60.
Wang 2013 {published data only}
-
- Wang Y. Efficacy of olanzapine combined with magnesium valproate in the treatment of schizophrenia [奥氮平合用丙戊酸镁缓释片治疗精神分裂症疗效分析]. Chinese Journal of General Practice [中华全科医学] 2013, issue 05:730+749.
Wang 2013a {published data only}
-
- Wang ML, Jiang TC. Clinical observation of risperidone combined with sodium valproate in the treatment of schizophrenia with impulse and aggressive behavior [利培酮联合丙戊酸钠治疗伴有冲动和攻击行为的精神分裂症患者的临床观察]. Clinical Rational Drug Use [临床合理用药杂志] 2013, issue 01:72‐3.
Wang 2014 {published data only}
-
- Wang GL, Fu PX, Li XY, Lin JX, Ji QS, Li ZJ. A comparative study of the efficacy of risperidone combined with valproate sodium on the aggressive behavior of the patients with schizophrenia [利培酮合并丙戊酸钠对精神分裂症患者攻击行为疗效的对照研究]. Chinese Journal of Drug Abuse Prevention and Treatment [中国药物滥用防治杂志] 2014; Vol. 20, issue 4:200‐2.
Wang 2014a {published data only}
-
- Wang ZB. The effect of adjuvant sodium valproate for schizophrenia with behavioral disorders [丙戊酸钠对精神分裂症行为紊乱的辅助治疗作用]. Medical Journal of Chinese People's Health [中国民康医学] 2014; Vol. 26, issue 22:57‐8.
Wen 2013 {published data only}
-
- Wen J, Li Y, Wang G. Clinical efficacy and safety of quetiapine combined with magnesium valproate in the treatment of schizophrenia with aggressive behavior [喹硫平合并丙戊酸镁缓释片治疗具有攻击行为精神分裂症的临床疗效及安全性探讨]. Medical Journal of Chinese Civil Administration [中国民康医学] 2013, issue 11:55‐6.
Wu 2013a {published data only}
-
- Wu J, Lin T. Efficacy of magnesium valproate combined with quetiapine in the treatment of refractory schizophrenia [丙戊酸镁联合喹硫平治疗难治性精神分裂症的疗效研究]. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease [实用心脑肺血管病杂志] 2013, issue 04:140‐1.
Wu 2013b {published data only}
-
- Wu X, Yang Q, Zhang L, Zhou S. A comparative study of olanzapine combined with magnesium valproate sustained‐release tablets in the treatment of patients with positive symptoms of schizophrenia [奥氮平联合丙戊酸镁缓释片治疗精神分裂症伴阳性症状患者的对照研究]. Medical Journal of West China [西部医学] 2013, issue 12:1842‐4.
Xiao 2013 {published data only}
-
- Xiao H. A retrospective study of risperidone combined with magnesium valproate in the treatment of schizophrenia [利培酮合并丙戊酸镁治疗精神分裂症用药现状回顾分析]. Nei Mongol Journal of Traditional Chinese Medicine [内蒙古中医药] 2013, issue 06:30‐1.
Xu 2002 {published data only}
-
- Xu HL, Wang Y. The effect of adjuvant sodium valproate on auditory hallucinations in schizophrenia [丙戊酸钠对精神分裂症幻听症状的辅助疗效]. Journal of Clinical Psychological Medicine [临床精神医学杂志] 2002; Vol. 12, issue 3:157‐8.
Xu 2013 {published data only}
-
- Xu LH, Huang WD, Li CH, Ban RY, Luo HF, Li ZM. Ziprasidone combined with low‐dose sodium valproate in the treatment and nursing of female refractory schizophrenia [齐拉西酮联合小剂量丙戊酸钠治疗女性难治性精神分裂症的观察及护理]. Journal of Aerospace Medicine [航空航天医学杂志] 2013, issue 09:1133‐5.
Xu 2014 {published data only}
-
- Xu BF, Lou YM, Cai XH. Effects of magnesium valproate sustained‐release tablets combined with clozapine in the treatment of female refractory schizophrenia [丙戊酸镁缓释片联合氯氮平治疗女性难治性精神分裂症的疗效观察]. Chinese Journal of Trauma and Disability Medicine [中国伤残医学] 2014; Vol. 22, issue 6:148‐9.
Zhang 2007a {published data only}
-
- Zhang S‐L, Wang C‐X, Wang L‐T. Therapeutic efficacy of combination of risperidone with Depakine for schizophrenia. Medical Journal of Qilu 2007;22(3):265‐6.
Zhang 2007b {published data only}
-
- Zhang DW, Dai J, Xie WF, Chen WT. Sodium valproate combined with EEG as an adjuvant therapy for schizophrenia [丙戊酸钠对伴脑电图异常的精神分裂症的辅助治疗作用]. Medical Journal of Chinese People's Health [中国民康医学] 2007; Vol. 19, issue 12:1049, 1051.
Zhang 2008 {published data only}
-
- Zhang X, Wang XM. Magnesium valproate combined with risperidone in the treatment of schizophrenia with negative symptoms [丙戊酸镁辅助利培酮治疗精神分裂症阴性症状的疗效观察]. Chinese Journal of Misdiagnostics [中国误诊学杂志] 2008; Vol. 18, issue 18:4361‐2.
Zhang 2012 {published data only}
-
- Zhang X, Ye CP, Liu WP. Efficacy of sodium valproate combined with haloperidol in the treatment of early schizophrenia [丙戊酸钠联合氟哌啶醇治疗早期精神分裂症的疗效观察]. Chinese and Foreign Medical Research [中外医学研究] 2012; Vol. 23:38‐9.
Zhou 2012 {published data only}
-
- Zhou YH, Zhong YF, Zeng DZ, Dai LJ. Efficacy of magnesium valproate sustained‐release tablets combined with ziprasidone in the treatment of schizophrenia with aggressive behavior [丙戊酸镁缓释片联合齐拉西酮治疗精神分裂症攻击行为的疗效观察]. The 10th National conference of Psychiatry of Chinese Medical Association 2012:1.
Zhou 2013 {published data only}
-
- Zhou LC, Nong YX, Chen QM. Effect and safety comparison of risperidone single therapy or combined valproate on excited restless of schizophrenia [利培酮单药或与丙戊酸钠联用对精神分裂症兴奋躁动的作用及安全性比较]. Journal of Clinical Psychological Medicine [临床精神医学杂志] 2013, issue 04:261‐2.
Zhou 2014 {published data only}
-
- Zhou W, Xie WJ, Hu YZ. Clinical observation of olanzapine combined sustained‐release magnesium valproate tablet treating male schizophrenic patients with aggressive behavior [奥氮平合并丙戊酸镁缓释片治疗伴攻击行为的男性精神分裂症临床观察]. Journal of Clinical Psychiatry [临床精神医学杂志] 2014; Vol. 24, issue 3:194‐6.
Zhu 2005a {published data only}
-
- Zhu CH, Fu R, Yang Y. A comparative study of sodium valproate combined with clonazepam for schizophrenic agitation [丙戊酸钠与氯硝西泮控制精神分裂症激越症状的对照研究]. Medical Journal of the Chinese People's Armed Police Forces [武警医学] 2005;16(12):934‐5.
Zhu 2014 {published data only}
-
- Zhu YT, Zeng DF. A comparative study of antipsychotic drugs combined with low‐dose sodium valproate in the treatment of schizophrenia [抗精神病药物联合小剂量丙戊酸镁治疗精神分裂症攻击行为的对照研究]. China Practical Medicine [中国实用医药] 2014; Vol. 9, issue 13:140‐1.
References to ongoing studies
NCT00167934 {published data only}
-
- NCT00167934. Metabolic effects of valproate and antipsychotic therapy. clinicaltrials.gov/ct2/show/NCT00167934 [Date Accessed: 9 September 2005].
NCT00306475 {published data only}
-
- NCT00306475. Does the addition of divalproex sodium ER to an atypical antipsychotic drug (APD) improve cognition and psychopathology in outpatients with schizophrenia (sch) or schizoaffective disorder (sad)?. clinicaltrials.gov/ct2/show/NCT00306475 [Date Accessed: 22 March 2006].
Norrie 2000 {published data only}
-
- Norrie PD. Valproate augmentation of antipsychotic therapy. International Journal of Neuropsychopharmacology 2000;3 (Suppl 1):S173.
Wang 2003 {published data only}
-
- Wang G. 60 week, randomized, double‐blind, placebo‐controlled trial of valproate added to risperidone in 200 treatment‐naive, first‐episode participants with schizophrenia. Stanley Foundation Research Programs 2003.
Additional references
Adams 2014
Altman 1996
Andreasen 1989
-
- Andreasen NC. Scale for the assessment of negative symptoms. British Journal of Psychiatry 1989;155:53‐8. - PubMed
Baethge 2003
-
- Baethge C. Long‐term treatment of schizoaffective disorder: review and recommendations. Pharmacopsychiatry 2003;36(2):45‐56. - PubMed
Bagnall 2000
Bailer 2012
-
- Bailer M. Chemical properties of antiepileptic drugs (AEDs). Advanced Drug Delivery Reviews 2012;64(10):887‐95. - PubMed
Balen 1999
-
- Balen RM, Procyshyn RM. Valproic acid for seizure prophylaxis during clozapine therapy: where's the evidence?. International Journal of Psychiatry in Clinical Practice 1999;3(4):249‐51. - PubMed
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA 1996;276:637‐9. - PubMed
Besag 2006
-
- Besag FMC, Berry D . Interactions between antiepileptic and antipsychotic drugs. Drug Safety 2006;29(2):95‐118. - PubMed
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. - PubMed
Carpenter 1994
-
- Carpenter WT, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. - PubMed
Carta 2003
-
- Carta, MG, Hardoy MC, Hardoy MJ, Grunze H, Carpiniello B. The clinical use of gabapentin in bipolar spectrum disorders. Journal of Affective Disorders June 2003;75(1):83‐91. - PubMed
Cheine 2003
-
- Cheine M, Ahonen J, Wahlbeck K. Beta‐blocker supplementation of standard drug treatment for schizophrenia (Cochrane Review). Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD000234] - DOI
Christison 1991
-
- Christison GW, Kirch DG, Wyatt RJ. When symptoms persist: choosing among alternative somatic treatments for schizophrenia. Schizophrenia Bulletin 1991;17:217‐45. - PubMed
Citrome 2000
-
- Citrome L, Levine L, Allingham B. Changes in use of valproate and other mood stabilizers for participants with schizophrenia from 1994 to 1998. Psychiatric Services 2000;51(5):634‐8. - PubMed
Crowhurst 2002
-
- Crowhurst N. Philosophy, care and treatment on the psychiatric intensive care unit: themes, trends and future practice. Journal of Psychiatric and Mental Health Nursing 2002;9(6):689‐95. - PubMed
Davey Smith 1998
Davis 2000
-
- Davis LL, Ryan W, Adinoff B, Petty F. Comprehensive review of the psychiatric uses of valproate. Journal of Clinical Psychopharmacology 2000;20(1):1‐17. - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Abstracts of 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Divine 1992
-
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' participant care behavior. Journal of General Internal Medicine 1992;7:623‐9. - PubMed
Dold 2012
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Essali 2010
Freeman 1998
-
- Freeman MP, Stoll AL. Mood stabilizer combinations: a review of safety and efficacy. The American Journal of Psychiatry 1998;155(1):12‐21. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. - PubMed
Gilbody 2000
Goodman 2006
-
- Goodman F, Glassman, P, Maglione M, Suttorp M. Drug Class Review on Antiepileptic Drugs in Bipolar Mood Disorder, Neuropathic Pain, and Fibromyalgia. Oregon Health & Science University, 2006.
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy U. ECDEU assessment manual for psychopharmacology. Early Clinical Drug Evaluation (ECDEU) Assessment Manual for Psychopharmacology. Washington DC: National Institute of Mental Health, 1976.
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hiller 1986
-
- Hiller W, Zerssen D, Mombour W, Wittchen HU. Inpatient Multidimensional Psychiatric Scale. Eine multidimensionale Skala zur systematischen Erfassung des psychopathologischen Befundes (Dt. Version). Manual. Weinheim: Beltz Test GmbH, 1986.
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986.
Konstantinos 2012
-
- Konstantinos N, Fountoulakis, Siegfried K, Ole A, Pierre B, Ahmed O, et al. Efficacy of pharmacotherapy in bipolar disorder: a report by the WPA section on pharmacopsychiatry. European Archives of Psychiatry and Clinical Neuroscience 2012;262(1):1‐48. - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] - PubMed
Leucht 2005b
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Leucht 2007a
Leucht 2007b
Leucht 2007c
Mallinckrodt 2004
-
- Mallinckrodt CH, Watkin JG, Molenberghs G, Carroll RJ. Choice of the primary analysis in longitudinal clinical trials. Pharmaceutical Statistics 2004;3:161‐9.
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Marvaha 2004
-
- Marvaha S, Johnson S. Schizophrenia and employment? A review. Social Psychiatry and Psychiatric Epidemiology 2004;39:337‐49. - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman D, CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;258:1987‐91. - PubMed
NIMH 1970
-
- National Institute of Mental Health. Clinical Global Impression. In: Guy W, Bonato RR editor(s). Manual for the ECDEU Assessment. 2nd Edition. NIMH, 1970.
NIMH 1991
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. ECDEU Assessment Manual for Psychopharmacology, Revised. National Institute of Mental Health, 1991.
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:790‐812.
Pajonk 2005
-
- Pajonk FG. Clinical trial design in schizophrenia: implications for clinical decisions. Current Opinion in Psychiatry 2005;18(6):692‐99. - PubMed
Palmer 2005
-
- Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia: a reexamination. Archives of General Psychiatry 2005;62(3):247‐53. - PubMed
Pantelis 1996
-
- Pantelis C, Barnes TRE. Drug strategies and treatment‐resistant schizophrenia. Australian and New Zealand Journal of Psychiatry 1996;20(1):20‐37. - PubMed
Perugi 2004
-
- Perugi G, Ruffolo G. The empirical basis of the use of antiepileptics in the treatment of bipolar disorder. Clinical Neuropsychiatry 2004;1(3):165‐74.
Schooler 1993
-
- Schooler NR, Keith SJ. Clinical research for the treatment of schizophrenia. Psychopharmacology Bulletin 1993;29:431‐46. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Simpson 1970
-
- Simpson M, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavia 1970;212:11‐9. - PubMed
Sommer 2012
Srisurapanont 2004
Stahl 2004
-
- Stahl, SM, Grady, MM. A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Current Medicinal Chemistry 2004;11(3):313‐27. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tharyan 2005
Tranulis 2006
-
- Tranulis C, Mouaffak F, Chouchana L, Stip E, Gourevitch R, Poirier MF, et al. Somatic augmentation strategies in clozapine resistance ‐ what facts?. Clinical Neuropharmacology 2006;29(1):34‐44. - PubMed
Tsuang 1978
-
- Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry 1978;35:153‐55. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):3‐92. [MEDLINE: ] - PubMed
Wahlbeck 2001
-
- Wahlbeck K, Tuunainen A, Ahokas A, Leucht S. Drop‐out rates in randomised antipsychotic drug trials. Psychopharmacology 2001;155:230‐3. - PubMed
Xia 2009
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7.
References to other published versions of this review
Leucht 2004
-
- Leucht S, White P, McGrath J, Kissling W. Lithium for schizophrenia revisited: a systematic review and meta‐analysis of randomized controlled trials. Journal of Clinical Psychiatry 2004;65:177‐86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


