European COMPARative Effectiveness research on blended Depression treatment versus treatment-as-usual (E-COMPARED): study protocol for a randomized controlled, non-inferiority trial in eight European countries
- PMID: 27488181
- PMCID: PMC4972947
- DOI: 10.1186/s13063-016-1511-1
European COMPARative Effectiveness research on blended Depression treatment versus treatment-as-usual (E-COMPARED): study protocol for a randomized controlled, non-inferiority trial in eight European countries
Abstract
Background: Effective, accessible, and affordable depression treatment is of high importance considering the large personal and economic burden of depression. Internet-based treatment is considered a promising clinical and cost-effective alternative to current routine depression treatment strategies such as face-to-face psychotherapy. However, it is not clear whether research findings translate to routine clinical practice such as primary or specialized mental health care. The E-COMPARED project aims to gain knowledge on the clinical and cost-effectiveness of blended depression treatment compared to treatment-as-usual in routine care.
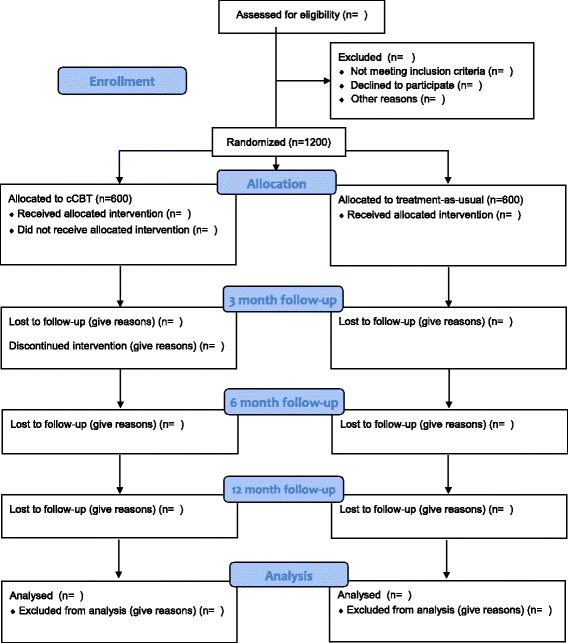
Methods/design: E-COMPARED will employ a pragmatic, multinational, randomized controlled, non-inferiority trial in eight European countries. Adults diagnosed with major depressive disorder (MDD) will be recruited in primary care (Germany, Poland, Spain, Sweden, and the United Kingdom) or specialized mental health care (France, The Netherlands, and Switzerland). Regular care for depression is compared to "blended" service delivery combining mobile and Internet technologies with face-to-face treatment in one treatment protocol. Participants will be followed up at 3, 6, and 12 months after baseline to determine clinical improvements in symptoms of depression (primary outcome: Patient Health Questionnaire-9), remission of depression, and cost-effectiveness. Main analyses will be conducted on the pooled data from the eight countries (n = 1200 in total, 150 participants in each country).
Discussion: The E-COMPARED project will provide mental health care stakeholders with evidence-based information and recommendations on the clinical and cost-effectiveness of blended depression treatment.
Trial registration: France: ClinicalTrials.gov NCT02542891 . Registered on 4 September 2015; Germany: German Clinical Trials Register DRKS00006866 . Registered on 2 December 2014; The Netherlands: Netherlands Trials Register NTR4962 . Registered on 5 January 2015; Poland: ClinicalTrials.Gov NCT02389660 . Registered on 18 February 2015; Spain: ClinicalTrials.gov NCT02361684 . Registered on 8 January 2015; Sweden: ClinicalTrials.gov NCT02449447 . Registered on 30 March 2015; Switzerland: ClinicalTrials.gov NCT02410616 . Registered on 2 April 2015; United Kingdom: ISRCTN registry, ISRCTN12388725 . Registered on 20 March 2015.
Keywords: Adults; Blended treatment; Cognitive behavioral treatment (CBT); Comparative effectiveness research (CER); Cost-effectiveness; Depression; Internet-based treatment; Randomized controlled trial (RCT); eHealth; iCBT.
Figures
Similar articles
-
Comparison of the Working Alliance in Blended Cognitive Behavioral Therapy and Treatment as Usual for Depression in Europe: Secondary Data Analysis of the E-COMPARED Randomized Controlled Trial.J Med Internet Res. 2024 May 31;26:e47515. doi: 10.2196/47515. J Med Internet Res. 2024. PMID: 38819882 Free PMC article. Clinical Trial.
-
Effectiveness of blended depression treatment for adults in specialised mental healthcare: study protocol for a randomised controlled trial.BMC Psychiatry. 2016 Apr 21;16:113. doi: 10.1186/s12888-016-0818-5. BMC Psychiatry. 2016. PMID: 27102812 Free PMC article. Clinical Trial.
-
Effectiveness and cost-effectiveness of a guided Internet- and mobile-based intervention for the indicated prevention of major depression in patients with chronic back pain-study protocol of the PROD-BP multicenter pragmatic RCT.BMC Psychiatry. 2017 Jan 21;17(1):36. doi: 10.1186/s12888-017-1193-6. BMC Psychiatry. 2017. PMID: 28109247 Free PMC article. Clinical Trial.
-
Interventions of computerized psychotherapies for depression in Primary Care in Spain.Actas Esp Psiquiatr. 2019 Nov;47(6):236-46. Epub 2019 Nov 1. Actas Esp Psiquiatr. 2019. PMID: 31869424 Review.
-
Clinical efficacy and economic evaluation of online cognitive behavioral therapy for major depressive disorder: a systematic review and meta-analysis.Expert Rev Pharmacoecon Outcomes Res. 2018 Feb;18(1):25-41. doi: 10.1080/14737167.2018.1407245. Epub 2017 Nov 30. Expert Rev Pharmacoecon Outcomes Res. 2018. PMID: 29145746 Review.
Cited by
-
Blended Psychological Therapy for the Treatment of Psychological Disorders in Adult Patients: Systematic Review and Meta-Analysis.Interact J Med Res. 2024 Oct 29;13:e49660. doi: 10.2196/49660. Interact J Med Res. 2024. PMID: 39470720 Free PMC article. Review.
-
From research to routine care: A historical review of internet-based cognitive behavioral therapy for adult mental health problems in Sweden.Digit Health. 2024 Oct 7;10:20552076241287059. doi: 10.1177/20552076241287059. eCollection 2024 Jan-Dec. Digit Health. 2024. PMID: 39381804 Free PMC article. Review.
-
Comparison of the Working Alliance in Blended Cognitive Behavioral Therapy and Treatment as Usual for Depression in Europe: Secondary Data Analysis of the E-COMPARED Randomized Controlled Trial.J Med Internet Res. 2024 May 31;26:e47515. doi: 10.2196/47515. J Med Internet Res. 2024. PMID: 38819882 Free PMC article. Clinical Trial.
-
Adherence to e-health interventions for substance use and the factors influencing it: Systematic Review, meta-analysis, and meta-regression.Digit Health. 2023 Sep 28;9:20552076231203876. doi: 10.1177/20552076231203876. eCollection 2023 Jan-Dec. Digit Health. 2023. PMID: 37780062 Free PMC article. Review.
-
Depression in Central and Eastern Europe: How Much It Costs? Cost of Depression in Romania.Healthcare (Basel). 2023 Mar 22;11(6):921. doi: 10.3390/healthcare11060921. Healthcare (Basel). 2023. PMID: 36981578 Free PMC article.
References
-
- Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev. 2011 - PubMed
-
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx B. Is excess mortality in depression generic or specific? A comprehensive meta-analysis of community and patient studies. Am J Psychiatry. 2014 - PubMed
-
- Smit F, Cuijpers P, Oostenbrink J, Batelaan N, de Graaf R, Beekman A. Costs of nine common mental disorders: implications for curative and preventive psychiatry. J Ment Health Policy Econ. 2006;9:193–200. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous