Development and Antiparkinsonian Activity of VU0418506, a Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor 4 Homomers without Activity at mGlu2/4 Heteromers
- PMID: 27441572
- PMCID: PMC5073817
- DOI: 10.1021/acschemneuro.6b00036
Development and Antiparkinsonian Activity of VU0418506, a Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor 4 Homomers without Activity at mGlu2/4 Heteromers
Abstract
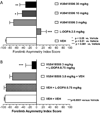
Metabotropic glutamate receptor 4 (mGlu4) is emerging as a potential therapeutic target for numerous central nervous system indications, including Parkinson's disease (PD). As the glutamate binding sites among the eight mGlu receptors are highly conserved, modulation of receptor activity via allosteric sites within the receptor transmembrane domains using positive and negative allosteric modulators (PAMs and NAMs, respectively) has become a common strategy. We and others have used PAMs targeting mGlu4 to show that potentiation of receptor signaling induces antiparkinsonian activity in a variety of PD animal models, including haloperidol-induced catalepsy and 6-hydroxydopamine-induced lesion. Recently, mGlu4 has been reported to form heteromeric complexes with other mGlu receptor subtypes, such as mGlu2, and the resulting heteromer exhibits a distinct pharmacological profile in response to allosteric modulators. For example, some mGlu4 PAMs do not appear to potentiate glutamate activity when mGlu2 and mGlu4 are coexpressed, whereas other compounds potentiate mGlu4 responses regardless of mGlu2 coexpression. We report here the discovery and characterization of VU0418506, a novel mGlu4 PAM with activity in rodent PD models. Using pharmacological approaches and Complemented Donor-Acceptor resonance energy transfer (CODA-RET) technology, we find that VU0418506 does not potentiate agonist-induced activity when mGlu2 and mGlu4 are heterodimerized, suggesting that the antiparkinsonian action of mGlu4 PAMs can be induced by compounds without activity at mGlu2/4 heteromers.
Keywords: Allosteric modulator; Parkinson’s disease; metabotropic glutamate receptor.
Figures


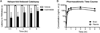
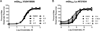
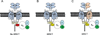

Similar articles
-
Elucidating the molecular logic of a metabotropic glutamate receptor heterodimer.Nat Commun. 2024 Oct 3;15(1):8552. doi: 10.1038/s41467-024-52822-4. Nat Commun. 2024. PMID: 39362861 Free PMC article.
-
The metabotropic glutamate receptor 4-positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine 2A antagonist in preclinical rodent models of Parkinson's disease.J Pharmacol Exp Ther. 2012 Feb;340(2):404-21. doi: 10.1124/jpet.111.187443. Epub 2011 Nov 16. J Pharmacol Exp Ther. 2012. PMID: 22088953 Free PMC article.
-
Synergy between L-DOPA and a novel positive allosteric modulator of metabotropic glutamate receptor 4: implications for Parkinson's disease treatment and dyskinesia.Neuropharmacology. 2013 Mar;66:158-69. doi: 10.1016/j.neuropharm.2012.03.022. Epub 2012 Apr 3. Neuropharmacology. 2013. PMID: 22491024
-
Targeting metabotropic glutamate receptors for novel treatments of schizophrenia.Mol Brain. 2017 Apr 26;10(1):15. doi: 10.1186/s13041-017-0293-z. Mol Brain. 2017. PMID: 28446243 Free PMC article. Review.
-
Metabotropic glutamate receptors as therapeutic targets in Parkinson's disease: An update from the last 5 years of research.Neuropharmacology. 2017 Mar 15;115:166-179. doi: 10.1016/j.neuropharm.2016.03.036. Epub 2016 Apr 4. Neuropharmacology. 2017. PMID: 27055772 Review.
Cited by
-
Elucidating the molecular logic of a metabotropic glutamate receptor heterodimer.Nat Commun. 2024 Oct 3;15(1):8552. doi: 10.1038/s41467-024-52822-4. Nat Commun. 2024. PMID: 39362861 Free PMC article.
-
Advances and Insights in Positron Emission Tomography Tracers for Metabotropic Glutamate Receptor 4 Imaging.J Med Chem. 2024 Jul 11;67(13):10517-10529. doi: 10.1021/acs.jmedchem.3c02431. Epub 2024 Jun 26. J Med Chem. 2024. PMID: 38924702 Review.
-
Pyrazolopyridine-based kinase inhibitors for anti-cancer targeted therapy.RSC Med Chem. 2024 Mar 25;15(5):1452-1470. doi: 10.1039/d4md00003j. eCollection 2024 May 22. RSC Med Chem. 2024. PMID: 38784451 Review.
-
Cholesterol in Class C GPCRs: Role, Relevance, and Localization.Membranes (Basel). 2023 Mar 3;13(3):301. doi: 10.3390/membranes13030301. Membranes (Basel). 2023. PMID: 36984688 Free PMC article.
-
A nanobody activating metabotropic glutamate receptor 4 discriminates between homo- and heterodimers.Proc Natl Acad Sci U S A. 2021 Aug 17;118(33):e2105848118. doi: 10.1073/pnas.2105848118. Proc Natl Acad Sci U S A. 2021. PMID: 34385321 Free PMC article.
References
-
- Schapira AH. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol. Sci. 2009;30:41–47. - PubMed
-
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat. Rev. Neurosci. 2005;6:787–798. - PubMed
-
- Marino MJ, Awad H, Poisik O, Wittmann M, Conn PJ. Localization and physiological roles of metabotropic glutamate receptors in the direct and indirect pathways of the basal ganglia. Amino Acids. 2002;23:185–191. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous


