Long-term follow-up of psilocybin-facilitated smoking cessation
- PMID: 27441452
- PMCID: PMC5641975
- DOI: 10.3109/00952990.2016.1170135
Long-term follow-up of psilocybin-facilitated smoking cessation
Erratum in
-
Corrigendum.Am J Drug Alcohol Abuse. 2017 Jan;43(1):127. doi: 10.1080/00952990.2016.1277105. Am J Drug Alcohol Abuse. 2017. PMID: 28106502 No abstract available.
Abstract
Background: A recent open-label pilot study (N = 15) found that two to three moderate to high doses (20 and 30 mg/70 kg) of the serotonin 2A receptor agonist, psilocybin, in combination with cognitive behavioral therapy (CBT) for smoking cessation, resulted in substantially higher 6-month smoking abstinence rates than are typically observed with other medications or CBT alone.
Objectives: To assess long-term effects of a psilocybin-facilitated smoking cessation program at ≥12 months after psilocybin administration.
Methods: The present report describes biologically verified smoking abstinence outcomes of the previous pilot study at ≥12 months, and related data on subjective effects of psilocybin.
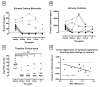
Results: All 15 participants completed a 12-month follow-up, and 12 (80%) returned for a long-term (≥16 months) follow-up, with a mean interval of 30 months (range = 16-57 months) between target-quit date (i.e., first psilocybin session) and long-term follow-up. At 12-month follow-up, 10 participants (67%) were confirmed as smoking abstinent. At long-term follow-up, nine participants (60%) were confirmed as smoking abstinent. At 12-month follow-up 13 participants (86.7%) rated their psilocybin experiences among the five most personally meaningful and spiritually significant experiences of their lives.
Conclusion: These results suggest that in the context of a structured treatment program, psilocybin holds considerable promise in promoting long-term smoking abstinence. The present study adds to recent and historical evidence suggesting high success rates when using classic psychedelics in the treatment of addiction. Further research investigating psilocybin-facilitated treatment of substance use disorders is warranted.
Keywords: Hallucinogen; addiction; mystical experience; nicotine; psilocybin; psychedelic; smoking cessation; spirituality; tobacco.
Figures
Comment in
-
It's time to take psilocybin seriously as a possible treatment for substance use disorders.Am J Drug Alcohol Abuse. 2017 Jan;43(1):4-6. doi: 10.1080/00952990.2016.1200060. Epub 2016 Aug 24. Am J Drug Alcohol Abuse. 2017. PMID: 27558746 No abstract available.
Similar articles
-
Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction.Curr Drug Abuse Rev. 2014;7(3):157-64. doi: 10.2174/1874473708666150107121331. Curr Drug Abuse Rev. 2014. PMID: 25563443 Free PMC article. Clinical Trial.
-
Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction.J Psychopharmacol. 2014 Nov;28(11):983-92. doi: 10.1177/0269881114548296. Epub 2014 Sep 11. J Psychopharmacol. 2014. PMID: 25213996 Free PMC article. Clinical Trial.
-
Psychedelic therapy for smoking cessation: Qualitative analysis of participant accounts.J Psychopharmacol. 2018 Jul;32(7):756-769. doi: 10.1177/0269881118780612. Epub 2018 Jun 25. J Psychopharmacol. 2018. PMID: 29938565
-
Classic Psychedelics in Addiction Treatment: The Case for Psilocybin in Tobacco Smoking Cessation.Curr Top Behav Neurosci. 2022;56:213-227. doi: 10.1007/7854_2022_327. Curr Top Behav Neurosci. 2022. PMID: 35704271 Review.
-
Potential Therapeutic Effects of Psilocybin.Neurotherapeutics. 2017 Jul;14(3):734-740. doi: 10.1007/s13311-017-0542-y. Neurotherapeutics. 2017. PMID: 28585222 Free PMC article. Review.
Cited by
-
Tobacco use disorder in patients with other mental disorders: a dual disorder perspective from clinical neuroscience.Front Psychiatry. 2024 Oct 11;15:1427561. doi: 10.3389/fpsyt.2024.1427561. eCollection 2024. Front Psychiatry. 2024. PMID: 39465048 Free PMC article. Review.
-
Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats.Mol Psychiatry. 2024 Oct 21. doi: 10.1038/s41380-024-02788-y. Online ahead of print. Mol Psychiatry. 2024. PMID: 39433903
-
Psychedelics: From Cave Art to 21st-Century Medicine for Addiction.Eur Addict Res. 2024;30(5):302-320. doi: 10.1159/000540062. Epub 2024 Sep 25. Eur Addict Res. 2024. PMID: 39321788 Free PMC article. Review.
-
Psychedelics as a potential treatment for tobacco use disorder: a systematic review.Discov Ment Health. 2024 Sep 17;4(1):37. doi: 10.1007/s44192-024-00095-0. Discov Ment Health. 2024. PMID: 39289250 Free PMC article. Review.
-
Epigenetic mechanisms of rapid-acting antidepressants.Transl Psychiatry. 2024 Sep 4;14(1):359. doi: 10.1038/s41398-024-03055-y. Transl Psychiatry. 2024. PMID: 39231927 Free PMC article. Review.
References
-
- World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. Geneva: World Health Organization; 2011.
-
- Mottillo S, Filion KB, Bélisle P, Joseph L, Gervais A, O’Loughlin J, Paradis G, Pihl R, Pilote L, Rinfret S, Tremblay M. Behavioural interventions for smoking cessation: a meta-analysis of randomized controlled trials. Eur Heart J. 2009 Mar 1;30(6):718–30. doi: 10.1093/eurheartj/ehn552. - DOI - PubMed
-
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Rockville, MD: Humana Press; 1992. pp. 207–224. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

