Contribution of ammonia oxidation to chemoautotrophy in Antarctic coastal waters
- PMID: 27187795
- PMCID: PMC5113851
- DOI: 10.1038/ismej.2016.61
Contribution of ammonia oxidation to chemoautotrophy in Antarctic coastal waters
Abstract
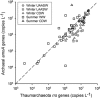
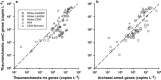
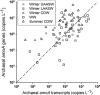
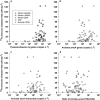
There are few measurements of nitrification in polar regions, yet geochemical evidence suggests that it is significant, and chemoautotrophy supported by nitrification has been suggested as an important contribution to prokaryotic production during the polar winter. This study reports seasonal ammonia oxidation (AO) rates, gene and transcript abundance in continental shelf waters west of the Antarctic Peninsula, where Thaumarchaeota strongly dominate populations of ammonia-oxidizing organisms. Higher AO rates were observed in the late winter surface mixed layer compared with the same water mass sampled during summer (mean±s.e.: 62±16 versus 13±2.8 nm per day, t-test P<0.0005). AO rates in the circumpolar deep water did not differ between seasons (21±5.7 versus 24±6.6 nm per day; P=0.83), despite 5- to 20-fold greater Thaumarchaeota abundance during summer. AO rates correlated with concentrations of Archaea ammonia monooxygenase (amoA) genes during summer, but not with concentrations of Archaea amoA transcripts, or with ratios of Archaea amoA transcripts per gene, or with concentrations of Betaproteobacterial amoA genes or transcripts. The AO rates we report (<0.1-220 nm per day) are ~10-fold greater than reported previously for Antarctic waters and suggest that inclusion of Antarctic coastal waters in global estimates of oceanic nitrification could increase global rate estimates by ~9%. Chemoautotrophic carbon fixation supported by AO was 3-6% of annualized phytoplankton primary production and production of Thaumarchaeota biomass supported by AO could account for ~9% of the bacterioplankton production measured in winter. Growth rates of thaumarchaeote populations inferred from AO rates averaged 0.3 per day and ranged from 0.01 to 2.1 per day.
Figures

Similar articles
-
Ammonia-oxidizing Archaea in the Arctic Ocean and Antarctic coastal waters.Environ Microbiol. 2009 Sep;11(9):2434-45. doi: 10.1111/j.1462-2920.2009.01974.x. Epub 2009 Jul 6. Environ Microbiol. 2009. PMID: 19601959
-
Abundance, diversity, and activity of ammonia-oxidizing prokaryotes in the coastal Arctic ocean in summer and winter.Appl Environ Microbiol. 2011 Mar;77(6):2026-34. doi: 10.1128/AEM.01907-10. Epub 2011 Jan 14. Appl Environ Microbiol. 2011. PMID: 21239542 Free PMC article.
-
Diversity, abundance, and activity of ammonia-oxidizing bacteria and archaea in Chongming eastern intertidal sediments.Appl Microbiol Biotechnol. 2013 Sep;97(18):8351-63. doi: 10.1007/s00253-012-4512-3. Epub 2012 Oct 30. Appl Microbiol Biotechnol. 2013. PMID: 23108528
-
Activity, abundance and diversity of nitrifying archaea and bacteria in the central California Current.Environ Microbiol. 2010 Jul;12(7):1989-2006. doi: 10.1111/j.1462-2920.2010.02205.x. Epub 2010 Mar 23. Environ Microbiol. 2010. PMID: 20345944
-
Ammonia oxidizers in the sea-surface microlayer of a coastal marine inlet.PLoS One. 2018 Aug 20;13(8):e0202636. doi: 10.1371/journal.pone.0202636. eCollection 2018. PLoS One. 2018. PMID: 30125317 Free PMC article.
Cited by
-
Genomic insight into strategy, interaction and evolution of nitrifiers in metabolizing key labile-dissolved organic nitrogen in different environmental niches.Front Microbiol. 2023 Dec 13;14:1273211. doi: 10.3389/fmicb.2023.1273211. eCollection 2023. Front Microbiol. 2023. PMID: 38156017 Free PMC article.
-
Transcriptomic Insights into Archaeal Nitrification in the Amundsen Sea Polynya, Antarctica.J Microbiol. 2023 Nov;61(11):967-980. doi: 10.1007/s12275-023-00090-0. Epub 2023 Dec 7. J Microbiol. 2023. PMID: 38062325
-
Vertical segregation and phylogenetic characterization of archaea and archaeal ammonia monooxygenase gene in the water column of the western Arctic Ocean.Extremophiles. 2023 Sep 5;27(3):24. doi: 10.1007/s00792-023-01310-6. Extremophiles. 2023. PMID: 37668803
-
Carbon content, carbon fixation yield and dissolved organic carbon release from diverse marine nitrifiers.Limnol Oceanogr. 2023 Jan;68(1):84-96. doi: 10.1002/lno.12252. Epub 2022 Oct 27. Limnol Oceanogr. 2023. PMID: 37064272 Free PMC article.
-
The spatial distribution and biogeochemical drivers of nitrogen cycle genes in an Antarctic desert.Front Microbiol. 2022 Oct 6;13:927129. doi: 10.3389/fmicb.2022.927129. eCollection 2022. Front Microbiol. 2022. PMID: 36274733 Free PMC article.
References
-
- Alonso-Sáez L, Andersson A, Heinrich F, Bertilsson S. (2011). High archaeal diversity in Antarctic circumpolar deep waters. Environ Microbiol Rep 3: 689–697. - PubMed
-
- Alonso-Sáez L, Sánchez O, Gasol JM, Balagué V, Pedrós-Alio C. (2008). Winter-to-summer changes in the composition and single-cell activity of near-surface Arctic prokaryotes. Environ Microbiol 10: 2444–2454. - PubMed
-
- Arrigo KR, Worthen D, Schnell A, Lizotte MP. (1998). Primary production in Southern Ocean waters. J Geophys Res 103: 15587–15600.
-
- Azam F, Fenchel T, Field JG, Gray JS, Meyer-Riel LA, Thingstad F. (1983). Ecological role of water column microbes in the sea. Mar Ecol Progr Ser 10: 257–263.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources


