Systemic and topical antibiotics for chronic rhinosinusitis
- PMID: 27113482
- PMCID: PMC8763400
- DOI: 10.1002/14651858.CD011994.pub2
Systemic and topical antibiotics for chronic rhinosinusitis
Abstract
Background: This review is one of six looking at the primary medical management options for patients with chronic rhinosinusitis.Chronic rhinosinusitis is common and is characterised by inflammation of the lining of the nose and paranasal sinuses leading to nasal blockage, nasal discharge, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. Systemic and topical antibiotics are used with the aim of eliminating infection in the short term (and some to reduce inflammation in the long term), in order to normalise nasal mucus and improve symptoms.
Objectives: To assess the effects of systemic and topical antibiotics in people with chronic rhinosinusitis.
Search methods: The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; CENTRAL (2015, Issue 8); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 29 September 2015.
Selection criteria: Randomised controlled trials (RCTs) with a follow-up period of at least three months comparing systemic or topical antibiotic treatment to (a) placebo or (b) no treatment or (c) other pharmacological interventions.
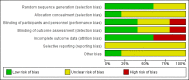
Data collection and analysis: We used the standard methodological procedures expected by Cochrane. Our primary outcomes were disease-specific health-related quality of life (HRQL), patient-reported disease severity and the commonest adverse event - gastrointestinal disturbance. Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score and the adverse events of suspected allergic reaction (rash or skin irritation) and anaphylaxis or other very serious reactions. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
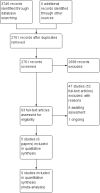
Main results: We included five RCTs (293 participants), all of which compared systemic antibiotics with placebo or another pharmacological intervention.The varying study characteristics made comparison difficult. Four studies recruited only adults and one only children. Three used macrolide, one tetracycline and one a cephalosporin-type antibiotic. Three recruited only patients with chronic rhinosinusitis without nasal polyps, one recruited patients with chronic rhinosinusitis with nasal polyps and one had a mixed population. Three followed up patients for 10 to 12 weeks after treatment had finished. Systemic antibiotics versus placebo Three studies compared antibiotics with placebo (176 participants).One study (64 participants, without polyps) reported disease-specific HRQL using the SNOT-20 (0 to 5, 0 = best quality of life). At the end of treatment (three months) the SNOT-20 score was lower in the group receiving macrolide antibiotics than the placebo group (mean difference (MD) -0.54 points, 95% confidence interval (CI) -0.98 to -0.10), corresponding to a moderate effect size favouring antibiotics (moderate quality evidence). Three months after treatment, it is uncertain if there was a difference between groups.One study (33 participants, with polyps) provided information on gastrointestinal disturbances and suspected allergic reaction (rash or skin irritation) after a short course of tetracycline antibiotic compared with placebo. We are very uncertain if antibiotics were associated with an increase in gastrointestinal disturbances (risk ratio (RR) 1.36, 95% CI 0.22 to 8.50) or skin irritation (RR 6.67, 95% CI 0.34 to 128.86) (very low quality evidence). Systemic antibiotics plus saline irrigation and intranasal corticosteroids versus placebo plus saline irrigation and intranasal corticosteroids One study (60 participants, some with and some without polyps) compared a three-month course of macrolide antibiotic with placebo; all participants also used saline irrigation and 70% used intranasal corticosteroids. Disease-specific HRQL was reported using SNOT-22 (0 to 110, 0 = best quality of life). Data were difficult to interpret (highly skewed and baseline imbalances) and it is unclear if there was an important difference at any time point (low quality evidence). To assess patient-reported disease severity participants rated the effect of treatment on a five-point scale (-2 for "desperately worse" to 2 for "cured") at the end of treatment (three months). For improvement in symptoms there was no difference between the antibiotics and placebo groups; the RR was 1.50 (95% CI 0.81 to 2.79; very low quality evidence), although there were also slightly more people who felt worse after treatment in the antibiotics group. There was no demonstrable difference in the rate of gastrointestinal disturbances between the groups (RR 1.07, 95% CI 0.16 to 7.10). General HRQL was measured using the SF-36. The authors stated that there was no difference between groups at the end of treatment (12 weeks) or two weeks later. Systemic antibiotics versus intranasal corticosteroids One study (43 participants, without polyps) compared a three-month course of macrolide antibiotic with intranasal corticosteroids. Patient-reported disease severity was assessed using a composite symptom score (0 to 40; 0 = no symptoms). It is very uncertain if there was a difference as patient-reported disease severity was similar between groups (MD -0.32, 95% CI -2.11 to 1.47; low quality evidence). Systemic antibiotics versus oral corticosteroids One study (28 participants, with polyps) compared a short course of tetracycline antibiotic (unclear duration, ˜20 days) with a 20-day course of oral corticosteroids. We were unable to extract data on any of the primary efficacy outcomes. It is uncertain if there was a difference ingastrointestinal disturbances (RR 1.00, 95% CI 0.16 to 6.14) or skin irritation (RR 2.00, 95% CI 0.20 to 19.62) as the results for these outcomes were similar between groups (very low quality evidence).
Authors' conclusions: We found very little evidence that systemic antibiotics are effective in patients with chronic rhinosinusitis. We did find moderate quality evidence of a modest improvement in disease-specific quality of life in adults with chronic rhinosinusitis without polyps receiving three months of a macrolide antibiotic. The size of improvement was moderate (0.5 points on a five-point scale) and only seen at the end of the three-month treatment; by three months later no difference was found.Despite a general understanding that antibiotics can be associated with adverse effects, including gastrointestinal disturbances, the results in this review were very uncertain because the studies were small and few events were reported.No RCTs of topical antibiotics met the inclusion criteria.More research in this area, particularly evaluating longer-term outcomes and adverse effects, is required.
Conflict of interest statement
Lee Yee Chong: none known.
Karen Head: none known.
Patorn Piromchai: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of the Cochrane ENT Group, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of the Cochrane ENT Group, but had no role in the editorial process for this review.
Figures













Update of
- doi: 10.1002/14651858.CD011994
Similar articles
-
Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis.Cochrane Database Syst Rev. 2016 Apr 26;4(4):CD011996. doi: 10.1002/14651858.CD011996.pub2. Cochrane Database Syst Rev. 2016. PMID: 27115217 Free PMC article. Review.
-
Short-course oral steroids alone for chronic rhinosinusitis.Cochrane Database Syst Rev. 2016 Apr 26;4(4):CD011991. doi: 10.1002/14651858.CD011991.pub2. Cochrane Database Syst Rev. 2016. PMID: 27113367 Free PMC article. Review.
-
Short-course oral steroids as an adjunct therapy for chronic rhinosinusitis.Cochrane Database Syst Rev. 2016 Apr 26;4(4):CD011992. doi: 10.1002/14651858.CD011992.pub2. Cochrane Database Syst Rev. 2016. PMID: 27115214 Free PMC article. Review.
-
Saline irrigation for chronic rhinosinusitis.Cochrane Database Syst Rev. 2016 Apr 26;4(4):CD011995. doi: 10.1002/14651858.CD011995.pub2. Cochrane Database Syst Rev. 2016. PMID: 27115216 Free PMC article. Review.
-
Different types of intranasal steroids for chronic rhinosinusitis.Cochrane Database Syst Rev. 2016 Apr 26;4(4):CD011993. doi: 10.1002/14651858.CD011993.pub2. Cochrane Database Syst Rev. 2016. PMID: 27115215 Free PMC article. Review.
Cited by
-
Protocol for a systematic review and meta-analysis of interventions aimed at delabeling low-risk penicillin allergies with consideration for sex and gender.Syst Rev. 2024 Oct 14;13(1):259. doi: 10.1186/s13643-024-02671-5. Syst Rev. 2024. PMID: 39402648 Free PMC article.
-
Inter-societal Delphi Consensus on the topical nasal treatments in Italy.Multidiscip Respir Med. 2024 Sep 4;19(1):991. doi: 10.5826/mrm.2024.991. Multidiscip Respir Med. 2024. PMID: 39229922 Free PMC article.
-
Targeting Epithelium Dysfunction and Impaired Nasal Biofilms to Treat Immunological, Functional, and Structural Abnormalities of Chronic Rhinosinusitis.Int J Mol Sci. 2023 Aug 3;24(15):12379. doi: 10.3390/ijms241512379. Int J Mol Sci. 2023. PMID: 37569753 Free PMC article. Review.
-
Exploring Endotypes in Chronic Rhinosinusitis (ExpRess): Protocol for a cohort study.PLoS One. 2023 Aug 2;18(8):e0289407. doi: 10.1371/journal.pone.0289407. eCollection 2023. PLoS One. 2023. PMID: 37531384 Free PMC article.
-
Treatment Strategy of Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: A Review of Recent Evidence.Int J Mol Sci. 2023 Mar 6;24(5):5015. doi: 10.3390/ijms24055015. Int J Mol Sci. 2023. PMID: 36902445 Free PMC article. Review.
References
References to studies included in this review
Otten 1994 {published data only}
-
- Otten HW, Antvelink JB, Ruyter de Wildt H, Rietema SJ, Siemelink RJ, Hordijk GJ. Is antibiotic treatment of chronic sinusitis effective in children?. Clinical Otolaryngology and Allied Sciences 1994;19(3):215‐7. - PubMed
Van Zele 2010 {published data only}
-
- Gevaert P, Zele T, Hellings P, Mayr S, Beule A, Ebbens F, et al. Doxycycline reduces ECP, MMP9 and nasal polyp size, in a double‐blind, randomized, placebo controlled, multicenter trial. World Allergy Organization Journal 2007;1 Suppl 3:S322.
-
- Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. Journal of Allergy and Clinical Immunology 2010;125(5):1069‐76.e4. - PubMed
Videler 2011 {published data only}
-
- Videler WJ, Badia, L, Harvey RJ, Gane S, Georgalas C, Meulen FW, et al. Lack of efficacy of long‐term, low‐dose azithromycin in chronic rhinosinusitis: a randomized controlled trial. Allergy 2011;66:1457‐68. - PubMed
Wallwork 2006 {published data only}
-
- Wallwork B, Coman W, Mackay‐Sim A, Greiff L, Cervin A. A double‐blind, randomized, placebo‐controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope 2006;116(2):189‐93. - PubMed
Zeng 2011 {published data only}
-
- Zeng M, Long X‐B, Cui Y‐H, Liu Z. Comparison of efficacy of mometasone furoate versus clarithromycin in the treatment of chronic rhinosinusitis without nasal polyps in Chinese adults. American Journal of Rhinology & Allergy 2011;25(6):203‐7. - PubMed
References to studies excluded from this review
Agbim 1975 {published data only}
-
- Agbim OG. A comparison trial of doxycycline and ampicillin in the treatment of acute sinusitis. Chemotherapy 1975;21(Suppl 1):68‐75. - PubMed
Amali 2015 {published data only}
-
- Amali A, Saedi B, Rahavi‐Ezabadi S, Ghazavi H, Hassanpoor N. Long‐term postoperative azithromycin in patients with chronic rhinosinusitis: a randomized clinical trial. American Journal of Rhinology & Allergy 2015;29(6):421‐4. - PubMed
Amini 2009 {published data only}
-
- Amini M, Yarmohammadi M, Izadi P. A comparing study of clarithromycin XL with co‐amoxiclav for treatment of chronic sinusitis: a clinical trial. Iranian Journal of Clinical Infectious Diseases 2009;4(4):197‐201.
Ansari 2015 {published data only}
-
- Ansari NN, Naghdi S, Fathali M, Bartley J, Rastak MS. A randomized clinical trial comparing pulsed ultrasound and erythromycin phonophoresis in the treatment of patients with chronic rhinosinusitis. Physiotherapy Theory & Practice 2015;31(3):166‐72. - PubMed
Artigas 1989 {published data only}
-
- Artigas R, Iborra M, Ridao M, Ridao M. Study of the relative efficacy between amoxicillin and amoxicillin‐clavulanate in chronic sinusitis in pediatric age. Revista Espanola de Alergologia e Inmunologia Clinica 1989;4 Suppl 1:59.
Beloborodova 1998 {published data only}
-
- Beloborodova NV, Sorokin GV. Cost‐effect clinical and pharmacological efficacy of amoxicillin clavulanate (Amoxyclav) in pediatric otorhinolaryngology. Russian Bulletin of Perinatology and Pediatrics 1998;5(43):49‐56.
Bezerra 2014 {published data only}
-
- Bezerra T, Soter AC, Pezato R, Pinna F, Bachert C, Zele T, et al. Long‐term low‐dose doxycycline for difficult‐to‐treat chronic rhinosinusitis with polyps. Otolaryngology ‐ Head and Neck Surgery 2014;151(1S):121‐2. - PubMed
Bobacheva 2012 {published data only}
-
- Bobacheva TI. The treatment of chronic polypous rhinosinusitis in the postoperative period with low doses of clarithromycin. Vestnik Otorinolaringologii 2012;4:68‐72. - PubMed
Bonfils 2015 {published data only}
-
- Bonfils P, Escabasse V, Coste A, Gilain L, Louvrier C, Serrano E, et al. Efficacy of tobramycin aerosol in nasal polyposis. European Annals of Otorhinolaryngology, Head and Neck Diseases 2015;132(3):119‐23. - PubMed
Chatzimanolis 1998 {published data only}
-
- Chatzimanolis E, Marsan N, Lefatzis D, Pavlopoulos A. Comparison of roxithromycin with co‐amoxiclav in patients with sinusitis. Journal of Antimicrobial Chemotherapy 1998;41 Suppl B:81‐4. - PubMed
Dellamonica 1994 {published data only}
-
- Dellamonica P, Choutet P, Lejeune JM, Lucht F, Morgon A, Pessey JJ, et al. Efficacy and tolerance of cefotiam hexetil in the super‐infected chronic sinusitis. A randomized, double‐blind study in comparison with cefixime. Annales d Oto‐Laryngologie et de Chirurgie Cervico‐Faciale 1994;111(4):217‐22. - PubMed
Desrosiers 2001 {published data only}
-
- Desrosiers MY, Salas‐Prato M. Treatment of chronic rhinosinusitis refractory to other treatments with topical antibiotic therapy delivered by means of a large‐particle nebulizer: results of a controlled trial. Otolaryngology ‐ Head and Heck Surgery 2001;125(3):265‐9. - PubMed
Edelstein 1993 {published data only}
-
- Edelstein DR, Avner SE, Chow JM, Duerksen RL, Johnson J, Ronis M, et al. Once‐a‐day therapy for sinusitis: a comparison study of cefixime and amoxicillin. Laryngoscope 1993;103(1 Pt 1):33‐41. - PubMed
El'kun 1999 {published data only}
-
- El'kun GB. Effectiveness of maxaquin and tarivid in combined therapy of patients with chronic sinusitis. Vestnik Otorinolaringologii 1999;6:45‐6. - PubMed
Fan 2014 {published data only}
-
- Fan Y, Xu R, Hong H, Luo Q, Xia W, Ding M, et al. High and low doses of clarithromycin treatment are associated with different clinical efficacies and immunomodulatory properties in chronic rhinosinusitis. Journal of Laryngology & Otology 2014;128(3):236‐41. - PubMed
Hashiba 1997 {published data only}
-
- Hashiba M. Clinical effects of long‐term administration of macrolide antibiotics to patients with chronic paranasal sinusitis: results of past reports. Japanese Journal of Antibiotics 1997;50 Suppl A:5‐7. - PubMed
Haxel 2015 {published data only}
-
- Haxel BR, Clemens M, Karaiskaki N, Dippold U, Kettern L, Mann WJ. Controlled trial for long‐term low‐dose erythromycin after sinus surgery for chronic rhinosinusitis. Laryngoscope 2015;125(5):1048‐55. - PubMed
Hiratsuka 1996 {published data only}
-
- Hiratsuka Y, Takagi A, Tokuda Y, Oishi N, Nagata H. Effect of roxithromycin on chronic sinusitis after endonasal sinus surgery. Oto‐Rhino‐Laryngology Tokyo 1996;39(2 Suppl 1):112‐6.
Huck 1993 {published data only}
-
- Huck W, Reed BD, Nielsen RW, Ferguson RT, Gray DW, Lund GK, et al. Cefaclor vs amoxicillin in the treatment of acute, recurrent, and chronic sinusitis. Archives of Family Medicine 1993;2(5):497‐503. - PubMed
Husfeldt 1993 {published data only}
-
- Husfeldt P, Egede F, Nielsen PB. Antibiotic treatment of sinusitis in general practice. A double‐blind study comparing ofloxacin and erythromycin. European Archives of Oto‐Rhino‐Laryngology 1993;250 Suppl 1:S23‐5. - PubMed
IRCT201312299014N {unpublished data only}
-
- IRCT201312299014N. Effect of topical vancomycin versus placebo on prevention of sinonasal polyposis recurrence after FESS (Functional Endoscopic Sinus Surgery). apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201312299014N20 (accessed 28 January 2016).
Ishiura 1995 {published data only}
-
- Ishiura Y, Fujimura M, Saito M, Shibata K, Nomura M, Nakatsumi Y, et al. Additive effect of continuous low‐dose ofloxacin on erythromycin therapy for sinobronchial syndrome. Respiratory Medicine 1995;89(10):677‐84. - PubMed
Jareoncharsri 2004 {published data only}
-
- Jareoncharsri P, Bunnag C, Fooanant S, Tunsuriyawong P, Voraprayoon S, Srifuengfung S, et al. An open label, randomized comparative study of levofloxacin and amoxicillin/clavulanic acid in the treatment of purulent sinusitis in adult Thai patients. Rhinology 2004;42(1):23‐9. - PubMed
Jervis‐Bardy 2012 {published data only}
-
- Jervis‐Bardy J, Boase S, Psaltis A, Foreman A, Wormald P. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope 2012;122(10):2148‐53. - PubMed
Jiang 2008 {published data only}
-
- Jiang R‐S, Liang K‐L, Yang K‐Y, Shiao J‐Y, Su M‐C, Hsin C‐H, et al. Postoperative antibiotic care after functional endoscopic sinus surgery. American Journal of Rhinology 2008;22(6):608‐12. - PubMed
Kita 1995 {published data only}
-
- Kita H, Takezawa H, Isobe M, Kataura A. Long‐term low dose erythromycin (EM) and roxithromycin (RXM) therapy for chronic sinusitis. Practica Otologica, Supplement 1995;84:62‐9.
Korkmaz 2014 {published data only}
-
- Korkmaz H, Ocal B, Tatar EC, Tatar I, Ozdek A, Saylam G, et al. Biofilms in chronic rhinosinusitis with polyps: is eradication possible?. European Archives of Oto‐rhino‐laryngology 2014;271(10):2695‐702. - PubMed
Kunel'skaya 2008 {published data only}
-
- Kunel'skaya NL, Gurov AV, Kudriavtseva IS, Kafarskaia LI, Izotova GN. Study of the efficacy of cefixime (suprax) in patients with acute and recurrent chronic purulent sinusitis. Vestnik Otorinolaringologii 2008;6:55‐8. - PubMed
Legent 1994 {published data only}
-
- Legent F, Bordure P, Beauvillain C. Comparative study of ciprofloxacin and amoxicillin‐clavulanic acid in the treatment of chronic sinusitis [Etude comparative de la ciprofloxacine et de l'amoxicilline/acide clavulanique dans le traitement des sinusites chroniques]. Medecine et Maladies Infectieuses 1993;23(6):8‐13.
-
- Legent F, Bordure P, Beauvillain C, Berche P. A double‐blind comparison of ciprofloxacin and amoxycillin/clavulanic acid in the treatment of chronic sinusitis. Chemotherapy 1994;40 Suppl 1:8‐15. - PubMed
Li 2000 {published data only}
-
- Li XP, Qin ZM, Zheng RH, Tan QL, Zhou YY, Zhu L, et al. Comparison of the effectiveness of levofloxacin and cefuroxime for the treatment of sinusitis. Lin Chuang Erh Pi Yen Hou Ko Tsa Chih [Journal of Clinical Otorhinolaryngology] 2000;14(12):573‐5. - PubMed
Li 2002 {published data only}
-
- Li ZY, Liang YL. Comparison of effect of metronidazole combined with lincomycin and gentamycin combined with dexamethasone in treatment of chronic maxillary sinusitis. Zhongguo Xin Yao Yu Lin Chuang Za Zhi [Chinese Journal of New Drugs and Clinical Remedies] 2002;1:28‐9.
Li 2014 {published data only}
-
- Li Y, Wu T. A randomized controlled study of treating chronic rhinosinusitis with macrolides. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi [Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery] 2014;28(17):1289‐91. - PubMed
Mannhardt 1980 {published data only}
-
- Mannhardt J, Held J. Randomized comparative study of the therapeutic action of amoxicillin and doxycycline in sinusitis. Die Therapiewoche 1980;30(12):2087‐9.
Namyslowski 1998 {published data only}
-
- Namyslowski G, Misiolek M, Czecior E, Malafiej E, Orecka B, Namyslowski P, et al. Comparison of the efficacy and tolerability of amoxycillin/clavulanic acid 875 mg b.i.d. with cefuroxime 500 mg b.i.d. in the treatment of chronic and acute exacerbation of chronic sinusitis in adults. Journal of Chemotherapy (Florence, Italy) 2002;14(5):508‐17. - PubMed
-
- Namyslowski G, Misiolek M, Malafiej E, Czecior E, Orecka B, Woch G, et al. Randomized clinical trial comparing the efficacy and safety of Augmentin versus cefuroxime in the treatment of chronic sinusitis in adult patients. Medical Science Monitor 1998;4(3):551‐4.
NCT01825408 {unpublished data only}
-
- NCT01825408. Duration of antibiotic therapy as part of maximal medical therapy for chronic rhinosinusitis. clinicaltrials.gov/show/NCT01825408 (accessed 28 January 2016).
NCT02307825 {unpublished data only}
-
- NCT02307825. Azithromycin for patients with chronic rhinosinusitis failing medical and surgical therapy (AZI‐CRS). https://clinicaltrials.gov/show/NCT02307825 (accessed 28 January 2016).
Otten 1997 {published data only}
-
- Otten FW, Grote JJ. Otitis media with effusion and chronic upper respiratory tract infection in children: a randomized, placebo‐controlled clinical study. Laryngoscope 1990;100(6):627‐33. - PubMed
-
- Otten FWA. Conservative treatment of chronic maxillary sinusitis in children. Long‐term follow‐up. Acta Oto‐rhino‐laryngologica Belgica 1997;51(3):173‐5. - PubMed
-
- Otten FWA, Grote JJ. Treatment of chronic maxillary sinusitis in children. International Journal of Pediatric Otorhinolaryngology 1988;15(3):269‐78. - PubMed
Peric 2011 {published data only}
-
- Peric A, Vojvodic D, Vukomanovic‐Durdevic B. Nasal polyps with more intense epithelial eosinophil infiltration respond worse to long‐term low‐dose treatment by clarithromycin. Advances in Clinical and Experimental Medicine 2011;20(3):325‐34.
Portier 1996 {published data only}
-
- Portier H, Bonfils P, Chobaut JC, Lallemant JG, Martin C, Pessey JJ, et al. Clinical efficacy and safety of cefotiam hexetil for over infected chronic sinusitis. Comparison with amoxicillin/clavulanic acid. [French]. Medecine et Maladies Infectieuses 1996;26(12):1188‐93.
Rachelefsky 1982 {published data only}
-
- Rachelefsky GS, Katz RM, Siegel SC. Chronic sinusitis in children with respiratory allergy: the role of antimicrobials. Journal of Allergy and Clinical Immunology 1982;69(4):382‐7. - PubMed
Schalek 2009 {published data only}
-
- Schalek P, Petrás P, Klement V, Hahn A. Short‐term antibiotics treatment in patients with nasal polyps and enterotoxins producing Staphylococcus aureus strains. European Archives of Oto‐rhino‐laryngology 2009;266(12):1909‐13. - PubMed
Sreenath 2015 {published data only}
-
- Sreenath SB, Taylor RJ, Miller JD, Ambrose EC, Rawal RB, Ebert CS, et al. A prospective randomized cohort study evaluating 3 weeks vs 6 weeks of oral antibiotic treatment in the setting of "maximal medical therapy" for chronic rhinosinusitis. International Forum of Allergy and Rhinology 2015;5(9):820‐8. - PubMed
Sykes 1986 {published data only}
-
- Sykes DA, Wilson R, Chan KL, Mackay IS, Cole PJ. Relative importance of antibiotic and improved clearance in topical treatment of chronic mucopurulent rhinosinusitis. A controlled study. Lancet 1986;2(8503):359. - PubMed
Varvianskaia 2013 {published data only}
-
- Varvianskaia AV, Lopatin AS. The effectiveness of long‐term treatment of polypous rhinosinusitis with low doses of macrolides. Vestnik Otorinolaringologii 2013;5:22‐7. - PubMed
Videler 2008 {published data only}
-
- Videler WJM, Drunen CM, Reitsma JB, Fokkens W J. Nebulized bacitracin/colimycin: a treatment option in recalcitrant chronic rhinosinusitis with Staphylococcus aureus? A double‐blind, randomized, placebo‐controlled, cross‐over pilot study. Rhinology 2008;46(2):92‐8. - PubMed
Watanabe 2003 {published data only}
-
- Watanabe T, Ichinomiya K, Suzuki M. Combined therapy for patients with chronic paranasal sinusitis using a macrolide antibiotic and YAMIK catheter. Japanese Journal of Antibiotics 2003;56 Suppl A:154‐7. - PubMed
Wei 2011 {published data only}
-
- NCT00465530. Comparing the use of saline or saline plus gentamycin in nasal irrigation to treat chronic sinusitis in children. https://clinicaltrials.gov/show/NCT00465530 (accessed 28 January 2016).
-
- Wei JL, Sykes KJ, Johnson P, He J, Mayo MS. Safety and efficacy of once‐daily nasal irrigation for the treatment of pediatric chronic rhinosinusitis. Laryngoscope 2011;121(9):1989‐2000. - PubMed
References to studies awaiting assessment
Behm 2002 {published data only}
-
- Behm J, Corcoran G. Health resource utilization: moxifloxacin compared to levofloxacin and amoxicillin clavulanate in reducing "practice time use" in the treatment of sinusitis. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A107.
Jiang 2001 {published data only}
-
- Jiang RS, Wang JW. Bacteriology of chronic sinusitis after amoxicillin‐clavulanate potassium therapy. Otolaryngology ‐ Head and Neck Surgery 2001;124(6):683‐6. - PubMed
Kataoka 2003 {published data only}
-
- Kataoka S, Ogasawara K, Ishimitsu R, Tagami H, Iwamoto J, Kawauchi H. Combined drug therapy for patients with intractable paranasal sinusitis complicating allergic rhinitis. Japanese Journal of Antibiotics 2003;56 Suppl A:158‐61. - PubMed
Kim 2003 {published data only}
-
- Kim BG, Lee D‐M, Jo J‐H, Jung D‐G, Kang J‐M, Kim S‐W. Effect of roxithromycin and intranasal fluticasone spray in reducing symptoms of chronic sinusitis, polyp size and IL‐4 in allergic patients. Journal of Rhinology 2003;10(1):37‐41.
Ziuzio 1995 {published data only}
-
- Ziuzio S, Sakson B. Results of conservative treatment of chronic purulent sinusitis. Otolaryngologia Polska 1995;49 Suppl 23:178‐82. - PubMed
References to ongoing studies
EUCTR 2005 (2005‐004736‐51) {published data only}
-
- EUCTR 2005 (2005‐004736‐51). Double‐blind randomized placebo‐controlled multinational, multicentre‐trial on prolonged macrolide treatment in patients with moderate/severe chronic rhinosinusitis. clinicaltrialsregister.eu/ctr‐search/trial/2005‐004736‐51 accessed 28 January 2016.
Additional references
Balk 2012
-
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within‐arm correlation imputation in trials of continuous outcomes [Internet]. Report No.: 12(13)‐EHC141‐EF. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality, 2012. - PubMed
Bewick 2014
-
- Bewick JC, Egro FM, Masterson LM, Philpott CM. Preliminary findings: the feasibility study for a randomized controlled trial of clarithromycin in chronic rhinosinusitis. Otolaryngology ‐ Head and Neck Surgery 2014;15(Suppl 1):P25.
Bucher 2004
-
- Bucher HC, Tschudi P, Young J, Périat P, Welge‐Lüussen A, Züst H, et al. Effect of amoxicillin‐clavulanate in clinically diagnosed acute rhinosinusitis: a placebo‐controlled, double‐blind, randomized trial in general practice. Archives of Internal Medicine 2004;163(15):1793‐8. - PubMed
Cho 2012
Chong 2016a
Chong 2016b
Chong 2016c
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988.
DeMarcantonio 2011
-
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. - PubMed
Donath 2013
-
- Donath E, Chaudhry A, Hernandez‐Aya LF, Lit L. A meta‐analysis on the prophylactic use of macrolide antibiotics for the prevention of disease exacerbations in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2013;107:1385‐92. - PubMed
Ebbens 2010
-
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. - PubMed
Ebbens 2011
-
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. - PubMed
Egger 1997
EPOS 2007
-
- Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps. Rhinology. Supplement 2007;20:1‐136. - PubMed
EPOS 2012
-
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. - PubMed
Fokkens 2007
-
- Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps 2007. Rhinology 2007;45(Suppl 20):1‐139. - PubMed
Genoway 2011
-
- Genoway KA, Philpott CM, Javer AR. Pathogen yield and antimicrobial resistance patterns of chronic rhinosinusitis patients presenting to a tertiary rhinology centre. Journal of Otolaryngology ‐ Head & Neck Surgery 2011;40(3):232‐7. - PubMed
Gliklich 1995
-
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Haruna 2009
-
- Haruna S, Shimada C, Ozawa M, Fukami S, Moriyama H. A study of poor responders for long‐term, low‐dose macrolide administration for chronic sinusitis. Rhinology 2009;47(1):66‐71. - PubMed
Harvey 2009
-
- Harvey RJ, Wallwork BD, Lund VJ. Anti‐inflammatory effects of macrolides: applications in chronic rhinosinusitis. Immunology and Allergy Clinics of North America 2009;29(4):689‐703. - PubMed
Hastan 2011
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. - PubMed
Hatipoglu 2005
-
- Hatipoglu U, Rubinstein I. Treatment of chronic rhinosinusitis with low‐dose, long‐term macrolide antibiotics: an evolving paradigm. Current Allergy and Asthma Reports 2005;5:491‐4. - PubMed
Head 2016a
Head 2016b
Hopkins 2009
-
- Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22‐item Sinonasal Outcome Test. Clinical Otolaryngology 2009;34(5):447‐54. - PubMed
Hopkins 2016
-
- Hopkins C, Philpott C, Crowe S, Regan S, Degun A, Papachristou I, et al. Identifying the most important outcomes for systematic reviews of interventions for rhinosinusitis in adults: working with Patients, Public and Practitioners. Rhinology 2016; Vol. 54, issue 1:20‐6. [DOI: 10.4193/Rhin15.199] - DOI - PubMed
Inamura 2000
-
- Inamura K, Ohta N, Fukase S, Kasajima N, Aoyagi M. The effect of erythromycin on human peripheral neutrophil apoptosis. Rhinology 2000;38:124‐9. - PubMed
Iyer 2016
Jaeschke 1989
-
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407‐15. [PUBMED: 2691207] - PubMed
Kern 2008
Keswani 2012
Lanza 1997
-
- Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngology ‐ Head and Neck Surgery 1997;117:S1‐7. - PubMed
Larsen 2004
-
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. - PubMed
MacLaughlin 2000
-
- MacLaughlin EJ, Saseen JJ, Malone DC. Costs of beta‐lactam allergies: selection and costs of antibiotics for patients with a reported beta‐lactam allergy. Archives of Family Medicine 2000;9(8):722‐6. - PubMed
Miyanohara 2000
-
- Miyanohara T, Ushikai M, Matsune S, Ueno K, Katahira S, Kurono Y. Effects of clarithromycin on cultured human nasal epithelial cells and fibroblasts. Laryngoscope 2000;110:126‐31. - PubMed
Piromchai 2011
Pynnonen 2013
Ragab 2004
-
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. - PubMed
Ragab 2010
-
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. - PubMed
Revicki 2008
-
- Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient‐reported outcomes. Journal of Clinical Epidemiology 2008;61(2):102‐9. [PUBMED: 18177782] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shi 2014
-
- Shi ZL, Peng H, Hu XW, Hu JG. Effectiveness and safety of macrolides in bronchiectasis patients: a meta‐analysis and systematic review. Pulmonary Pharmacology and Therapeutics 2014;28(2):171‐8. - PubMed
Soler 2010
Tamaoki 2004
-
- Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. American Journal of Medicine 2004;117:5S‐11S. - PubMed
Tan 2011
Tomassen 2011
-
- Tomassen P, Zele T, Zhang N, Perez‐ Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. - PubMed
van Drunen 2009
-
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. - PubMed
Wozniak 2004
-
- Wozniak DJ, Keyser R. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest 2004;125:62S‐9S. - PubMed
Zhang 2008
-
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. - PubMed
Zhang 2009
-
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous


