Safety of Tdap vaccine in pregnant women: an observational study
- PMID: 27091823
- PMCID: PMC4838681
- DOI: 10.1136/bmjopen-2015-010911
Safety of Tdap vaccine in pregnant women: an observational study
Abstract
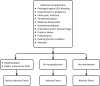
Objectives: Actively recruit and intensively follow pregnant women receiving a dose of acellular pertussis vaccine for 4 weeks after vaccination.
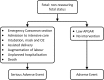
Design and settings: A prospective observational study conducted in 2 New Zealand regions.
Participants: Women in their 28th-38th week of pregnancy, recruited from primary care and antenatal clinics at the time of Tdap administration. Telephone interviews were conducted at 48 h and 4 weeks postvaccination.
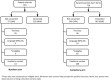
Main outcomes measures: Outcomes were injection site reactions, systemic symptoms and serious adverse events (SAEs). Where available, data have been classified and reported according to Brighton Collaboration definitions.
Results: 793 women participated with 27.9% receiving trivalent inactivated influenza vaccine concomitantly. 79% of participants reported mild or moderate pain and 2.6% severe pain. Any swelling was reported by 7.6%, induration by 12.0% (collected from 1 site only, n=326), and erythema by 5.8% of participants. Fever was reported by 17 (2.1%) participants, 14 of these occurred within 24 h. Headache, dizziness, nausea, myalgia or arthralgia was reported by <4% of participants, respectively, and fatigue by 8.4%. During the study period, there were 115 adverse events in 113 participants, most of which were minor. At the end of the reporting period, 31 events were classified as serious (eg, obstetric bleeding, hypertension, infection, tachycardia, preterm labour, exacerbation of pre-existing condition and pre-eclampsia). All had variable onset time from vaccination. There were two perinatal deaths. Clinician assessment of all SAEs found none likely to be vaccine related.
Conclusions: Vaccination with Tdap in pregnant women was well tolerated with no SAE likely to be caused by the vaccine.
Trial registration number: ACTRN12613001045707.
Keywords: acellular pertussis vaccine; pregnancy; safety; vaccination; whooping cough.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
Similar articles
-
Pertussis Immunisation in Pregnancy Safety (PIPS) Study: A retrospective cohort study of safety outcomes in pregnant women vaccinated with Tdap vaccine.Vaccine. 2018 Aug 16;36(34):5173-5179. doi: 10.1016/j.vaccine.2018.07.011. Epub 2018 Jul 18. Vaccine. 2018. PMID: 30031662
-
A randomised, double-blind, non-inferiority clinical trial on the safety and immunogenicity of a tetanus, diphtheria and monocomponent acellular pertussis (TdaP) vaccine in comparison to a tetanus and diphtheria (Td) vaccine when given as booster vaccinations to healthy adults.Vaccine. 2012 Aug 10;30(37):5464-71. doi: 10.1016/j.vaccine.2012.06.073. Epub 2012 Jul 6. Vaccine. 2012. PMID: 22776216 Clinical Trial.
-
Infant outcomes after exposure to Tdap vaccine in pregnancy: an observational study.BMJ Open. 2016 Jan 6;6(1):e009536. doi: 10.1136/bmjopen-2015-009536. BMJ Open. 2016. PMID: 26739731 Free PMC article.
-
Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2008 May 30;57(RR-4):1-51. MMWR Recomm Rep. 2008. PMID: 18509304 Review.
-
Influenza and tetanus, diphtheria, and acellular pertussis vaccinations during pregnancy.Obstet Gynecol Surv. 2012 Apr;67(4):251-7. doi: 10.1097/OGX.0b013e3182524cee. Obstet Gynecol Surv. 2012. PMID: 22495061 Review.
Cited by
-
The Present and Future Aspects of Life-Long Pertussis Prevention: Narrative Review with Regional Perspectives for Türkiye.Infect Dis Ther. 2023 Nov;12(11):2495-2512. doi: 10.1007/s40121-023-00876-0. Epub 2023 Oct 10. Infect Dis Ther. 2023. PMID: 37815753 Free PMC article. Review.
-
Safety of COVID-19 vaccines during pregnancy: A systematic review and meta-analysis.Vaccine. 2023 Jun 7;41(25):3688-3700. doi: 10.1016/j.vaccine.2023.03.038. Epub 2023 Mar 27. Vaccine. 2023. PMID: 37012114 Free PMC article. Review.
-
Safety and use of tetanus-diphtheria-acellular pertussis-5 (Tdap5) vaccine during pregnancy: findings from 11 years of reporting to a pregnancy registry.Hum Vaccin Immunother. 2021 Dec 2;17(12):5325-5333. doi: 10.1080/21645515.2021.1915038. Epub 2021 Dec 29. Hum Vaccin Immunother. 2021. PMID: 34965196 Free PMC article.
-
Comprehensive Overview of Vaccination during Pregnancy in Europe.J Pers Med. 2021 Nov 13;11(11):1196. doi: 10.3390/jpm11111196. J Pers Med. 2021. PMID: 34834548 Free PMC article. Review.
-
Maternal vaccination: a review of current evidence and recommendations.Am J Obstet Gynecol. 2022 Apr;226(4):459-474. doi: 10.1016/j.ajog.2021.10.041. Epub 2021 Nov 11. Am J Obstet Gynecol. 2022. PMID: 34774821 Free PMC article. Review.
References
-
- Advisory Committee on Immunization Practices (ACIP). Summary Report February 23–24, 2011. Atlanta, Georgia, 2011.
-
- Davies SC. Temporary programme of pertussis (whooping cough) vaccination of pregnant women. 28 Sept 2012 Department of Health, London, 2012.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical