An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic Aβ42 aggregates linked with Alzheimer's disease
- PMID: 26933687
- PMCID: PMC4758743
- DOI: 10.1126/sciadv.1501244
An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic Aβ42 aggregates linked with Alzheimer's disease
Abstract
The conversion of the β-amyloid (Aβ) peptide into pathogenic aggregates is linked to the onset and progression of Alzheimer's disease. Although this observation has prompted an extensive search for therapeutic agents to modulate the concentration of Aβ or inhibit its aggregation, all clinical trials with these objectives have so far failed, at least in part because of a lack of understanding of the molecular mechanisms underlying the process of aggregation and its inhibition. To address this problem, we describe a chemical kinetics approach for rational drug discovery, in which the effects of small molecules on the rates of specific microscopic steps in the self-assembly of Aβ42, the most aggregation-prone variant of Aβ, are analyzed quantitatively. By applying this approach, we report that bexarotene, an anticancer drug approved by the U.S. Food and Drug Administration, selectively targets the primary nucleation step in Aβ42 aggregation, delays the formation of toxic species in neuroblastoma cells, and completely suppresses Aβ42 deposition and its consequences in a Caenorhabditis elegans model of Aβ42-mediated toxicity. These results suggest that the prevention of the primary nucleation of Aβ42 by compounds such as bexarotene could potentially reduce the risk of onset of Alzheimer's disease and, more generally, that our strategy provides a general framework for the rational identification of a range of candidate drugs directed against neurodegenerative disorders.
Keywords: chemical kinetics; molecular mechanisms; neurodegeneration; protein misfolding.
Figures
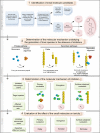


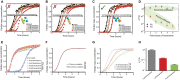
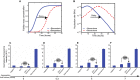
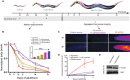
Similar articles
-
Systematic development of small molecules to inhibit specific microscopic steps of Aβ42 aggregation in Alzheimer's disease.Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):E200-E208. doi: 10.1073/pnas.1615613114. Epub 2016 Dec 23. Proc Natl Acad Sci U S A. 2017. PMID: 28011763 Free PMC article.
-
Menadione sodium bisulfite inhibits the toxic aggregation of amyloid-β(1-42).Biochim Biophys Acta Gen Subj. 2018 Oct;1862(10):2226-2235. doi: 10.1016/j.bbagen.2018.07.019. Epub 2018 Jul 20. Biochim Biophys Acta Gen Subj. 2018. PMID: 30036601
-
Trodusquemine enhances Aβ42 aggregation but suppresses its toxicity by displacing oligomers from cell membranes.Nat Commun. 2019 Jan 15;10(1):225. doi: 10.1038/s41467-018-07699-5. Nat Commun. 2019. PMID: 30644384 Free PMC article.
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
-
Small-molecule inhibitors/modulators of amyloid-β peptide aggregation and toxicity for the treatment of Alzheimer's disease: a patent review (2010 - 2012).Expert Opin Ther Pat. 2013 May;23(5):581-96. doi: 10.1517/13543776.2013.772983. Epub 2013 Feb 21. Expert Opin Ther Pat. 2013. PMID: 23425062 Review.
Cited by
-
Repurposing Anidulafungin for Alzheimer's Disease via Fragment-Based Drug Discovery.ACS Chem Neurosci. 2024 Aug 21;15(16):2995-3008. doi: 10.1021/acschemneuro.4c00150. Epub 2024 Aug 3. ACS Chem Neurosci. 2024. PMID: 39096284 Free PMC article.
-
CoVAMPnet: Comparative Markov State Analysis for Studying Effects of Drug Candidates on Disordered Biomolecules.JACS Au. 2024 May 28;4(6):2228-2245. doi: 10.1021/jacsau.4c00182. eCollection 2024 Jun 24. JACS Au. 2024. PMID: 38938816 Free PMC article.
-
Kinetic models reveal the interplay of protein production and aggregation.Chem Sci. 2024 May 10;15(22):8430-8442. doi: 10.1039/d4sc00088a. eCollection 2024 Jun 5. Chem Sci. 2024. PMID: 38846392 Free PMC article.
-
Protein mimetic 2D FAST rescues alpha synuclein aggregation mediated early and post disease Parkinson's phenotypes.Nat Commun. 2024 Apr 30;15(1):3658. doi: 10.1038/s41467-024-47980-4. Nat Commun. 2024. PMID: 38688913 Free PMC article.
-
The thermodynamics of neurodegenerative disease.Biophys Rev (Melville). 2024 Mar 20;5(1):011303. doi: 10.1063/5.0180899. eCollection 2024 Mar. Biophys Rev (Melville). 2024. PMID: 38525484 Review.
References
-
- Alzheimer’s Association, 2012 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 8, 131–168 (2012). - PubMed
-
- Chiti F., Dobson C. M., Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 (2006). - PubMed
-
- Dobson C. M., Protein folding and misfolding. Nature 426, 884–890 (2003). - PubMed
-
- Selkoe D. J., Folding proteins in fatal ways. Nature 426, 900–904 (2003). - PubMed
-
- Haass C., Selkoe D. J., Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


