Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana
- PMID: 26689363
- PMCID: PMC4841471
- DOI: 10.1038/nature16446
Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana
Abstract
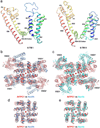
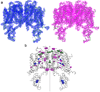
Two-pore channels (TPCs) contain two copies of a Shaker-like six-transmembrane (6-TM) domain in each subunit and are ubiquitously expressed in both animals and plants as organellar cation channels. Here we present the crystal structure of a vacuolar two-pore channel from Arabidopsis thaliana, AtTPC1, which functions as a homodimer. AtTPC1 activation requires both voltage and cytosolic Ca(2+). Ca(2+) binding to the cytosolic EF-hand domain triggers conformational changes coupled to the pair of pore-lining inner helices from the first 6-TM domains, whereas membrane potential only activates the second voltage-sensing domain, the conformational changes of which are coupled to the pair of inner helices from the second 6-TM domains. Luminal Ca(2+) or Ba(2+) can modulate voltage activation by stabilizing the second voltage-sensing domain in the resting state and shift voltage activation towards more positive potentials. Our Ba(2+)-bound AtTPC1 structure reveals a voltage sensor in the resting state, providing hitherto unseen structural insight into the general voltage-gating mechanism among voltage-gated channels.
Figures








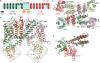
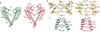


Similar articles
-
Voltage-gating and cytosolic Ca2+ activation mechanisms of Arabidopsis two-pore channel AtTPC1.Proc Natl Acad Sci U S A. 2021 Dec 7;118(49):e2113946118. doi: 10.1073/pnas.2113946118. Proc Natl Acad Sci U S A. 2021. PMID: 34845029 Free PMC article.
-
Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana.Nature. 2016 Mar 10;531(7593):258-62. doi: 10.1038/nature17194. Nature. 2016. PMID: 26961658 Free PMC article.
-
Gating of the two-pore cation channel AtTPC1 in the plant vacuole is based on a single voltage-sensing domain.Plant Biol (Stuttg). 2016 Sep;18(5):750-60. doi: 10.1111/plb.12478. Epub 2016 Jul 12. Plant Biol (Stuttg). 2016. PMID: 27270880
-
TPC1 vacuole SV channel gains further shape - voltage priming of calcium-dependent gating.Trends Plant Sci. 2023 Jun;28(6):673-684. doi: 10.1016/j.tplants.2023.01.001. Epub 2023 Feb 4. Trends Plant Sci. 2023. PMID: 36740491 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Structural biology and molecular pharmacology of voltage-gated ion channels.Nat Rev Mol Cell Biol. 2024 Nov;25(11):904-925. doi: 10.1038/s41580-024-00763-7. Epub 2024 Aug 5. Nat Rev Mol Cell Biol. 2024. PMID: 39103479 Review.
-
Plasticity of the selectivity filter is essential for permeation in lysosomal TPC2 channels.Proc Natl Acad Sci U S A. 2024 Aug 6;121(32):e2320153121. doi: 10.1073/pnas.2320153121. Epub 2024 Jul 29. Proc Natl Acad Sci U S A. 2024. PMID: 39074274 Free PMC article.
-
Dynamics of activation in the voltage-sensing domain of Ciona intestinalis phosphatase Ci-VSP.Nat Commun. 2024 Feb 15;15(1):1408. doi: 10.1038/s41467-024-45514-6. Nat Commun. 2024. PMID: 38360718 Free PMC article.
-
A charged existence: A century of transmembrane ion transport in plants.Plant Physiol. 2024 Apr 30;195(1):79-110. doi: 10.1093/plphys/kiad630. Plant Physiol. 2024. PMID: 38163639 Free PMC article. Review.
-
Identification of the CNGC Gene Family in Rice and Mining of Alleles for Application in Rice Improvement.Plants (Basel). 2023 Dec 6;12(24):4089. doi: 10.3390/plants12244089. Plants (Basel). 2023. PMID: 38140416 Free PMC article.
References
Methods References
-
- Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997;276(part A):307–326. Macromolecular Crystallography. - PubMed
-
- Vonrhein C, Blanc E, Roversi P, Bricogne G. Automated structure solution with autoSHARP. Methods Mol Biol. 2007;364:215–230. - PubMed
-
- Schneider TR, Sheldrick GM. Substructure solution with SHELXD. Acta Crystallogr D Biol Crystallogr. 2002;58:1772–1779. - PubMed
-
- Fortelle Edl, Bricogne G. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 1997;276(part A):472–494. Macromolecular Crystallography. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous


