Brugada Syndrome: Clinical, Genetic, Molecular, Cellular, and Ionic Aspects
- PMID: 26671757
- PMCID: PMC4737702
- DOI: 10.1016/j.cpcardiol.2015.06.002
Brugada Syndrome: Clinical, Genetic, Molecular, Cellular, and Ionic Aspects
Abstract
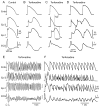
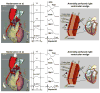
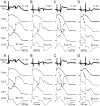
Brugada syndrome (BrS) is an inherited cardiac arrhythmia syndrome first described as a new clinical entity in 1992. Electrocardiographically characterized by distinct coved type ST segment elevation in the right-precordial leads, the syndrome is associated with a high risk for sudden cardiac death in young adults, and less frequently in infants and children. The electrocardiographic manifestations of BrS are often concealed and may be unmasked or aggravated by sodium channel blockers, a febrile state, vagotonic agents, as well as by tricyclic and tetracyclic antidepressants. An implantable cardioverter defibrillator is the most widely accepted approach to therapy. Pharmacologic therapy is designed to produce an inward shift in the balance of currents active during the early phases of the right ventricular action potential (AP) and can be used to abort electrical storms or as an adjunct or alternative to device therapy when use of an implantable cardioverter defibrillator is not possible. Isoproterenol, cilostazol, and milrinone boost calcium channel current and drugs like quinidine, bepridil, and the Chinese herb extract Wenxin Keli inhibit the transient outward current, acting to diminish the AP notch and thus to suppress the substrate and trigger for ventricular tachycardia or fibrillation. Radiofrequency ablation of the right ventricular outflow tract epicardium of patients with BrS has recently been shown to reduce arrhythmia vulnerability and the electrocardiographic manifestation of the disease, presumably by destroying the cells with more prominent AP notch. This review provides an overview of the clinical, genetic, molecular, and cellular aspects of BrS as well as the approach to therapy.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
Novel Therapeutic Strategies for the Management of Ventricular Arrhythmias Associated with the Brugada Syndrome.Expert Opin Orphan Drugs. 2015;3(6):633-651. doi: 10.1517/21678707.2015.1037280. Epub 2015 May 13. Expert Opin Orphan Drugs. 2015. PMID: 27559494 Free PMC article.
-
Therapy for the Brugada syndrome.Handb Exp Pharmacol. 2006;(171):305-30. doi: 10.1007/3-540-29715-4_12. Handb Exp Pharmacol. 2006. PMID: 16610350 Free PMC article. Review.
-
Recent advances in the treatment of Brugada syndrome.Expert Rev Cardiovasc Ther. 2018 Jun;16(6):387-404. doi: 10.1080/14779072.2018.1475230. Epub 2018 May 18. Expert Rev Cardiovasc Ther. 2018. PMID: 29757020 Free PMC article. Review.
-
Ionic and cellular mechanisms underlying the development of acquired Brugada syndrome in patients treated with antidepressants.J Cardiovasc Electrophysiol. 2012 Apr;23(4):423-32. doi: 10.1111/j.1540-8167.2011.02196.x. Epub 2011 Oct 28. J Cardiovasc Electrophysiol. 2012. PMID: 22034916 Free PMC article.
-
The management of Brugada syndrome patients.Cardiol J. 2007;14(1):97-106. Cardiol J. 2007. PMID: 18651443
Cited by
-
NaV1.5 autoantibodies in Brugada syndrome: pathogenetic implications.Eur Heart J. 2024 Oct 21;45(40):4336-4348. doi: 10.1093/eurheartj/ehae480. Eur Heart J. 2024. PMID: 39078224 Free PMC article.
-
Whole-heart computational modelling provides further mechanistic insights into ST-elevation in Brugada syndrome.Int J Cardiol Heart Vasc. 2024 Mar 4;51:101373. doi: 10.1016/j.ijcha.2024.101373. eCollection 2024 Apr. Int J Cardiol Heart Vasc. 2024. PMID: 38464963 Free PMC article.
-
Electrocardiographic temporo-spatial assessment of depolarization and repolarization changes after epicardial arrhythmogenic substrate ablation in Brugada syndrome.Eur Heart J Digit Health. 2023 Sep 6;4(6):473-487. doi: 10.1093/ehjdh/ztad050. eCollection 2023 Dec. Eur Heart J Digit Health. 2023. PMID: 38045442 Free PMC article.
-
Phase 2 Re-Entry Without Ito: Role of Sodium Channel Kinetics in Brugada Syndrome Arrhythmias.JACC Clin Electrophysiol. 2023 Dec;9(12):2459-2474. doi: 10.1016/j.jacep.2023.08.027. Epub 2023 Oct 11. JACC Clin Electrophysiol. 2023. PMID: 37831035 Free PMC article. Clinical Trial.
-
Noncoding RNAs and Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Cardiac Arrhythmic Brugada Syndrome.Cells. 2023 Oct 3;12(19):2398. doi: 10.3390/cells12192398. Cells. 2023. PMID: 37830612 Free PMC article. Review.
References
-
- Hermida JS, Lemoine JL, Aoun FB, Jarry G, Rey JL, Quiret JC. Prevalence of the brugada syndrome in an apparently healthy population. Am J Cardiol. 2000;86:91–94. - PubMed
-
- Postema PG. About Brugada syndrome and its prevalence. Europace. 2012;14:925–928. - PubMed
-
- Patel SS, Anees SS, Ferrick KJ. Prevalence of a Brugada pattern electrocardiogram in an urban population in the United States. Pacing Clin Electrophysiol. 2009;32:704–708. - PubMed
-
- Pecini R, Cedergreen P, Theilade S, Haunso S, Theilade J, Jensen GB. The prevalence and relevance of the Brugada-type electrocardiogram in the Danish general population: data from the Copenhagen City Heart Study. Europace. 1907;12:982–986. - PubMed
-
- Sinner MF, Pfeufer A, Perz S, Schulze-Bahr E, Monnig G, Eckardt L, Beckmann BM, Wichmann HE, Breithardt G, Steinbeck G, Fabritz L, Kaab S, Kirchhof P. Spontaneous Brugada electrocardiogram patterns are rare in the German general population: results from the KORA study. Europace. 2009;11:1338–1344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


