Use of Viremia to Evaluate the Baseline Case Fatality Ratio of Ebola Virus Disease and Inform Treatment Studies: A Retrospective Cohort Study
- PMID: 26625118
- PMCID: PMC4666644
- DOI: 10.1371/journal.pmed.1001908
Use of Viremia to Evaluate the Baseline Case Fatality Ratio of Ebola Virus Disease and Inform Treatment Studies: A Retrospective Cohort Study
Abstract
Background: The case fatality ratio (CFR) of Ebola virus disease (EVD) can vary over time and space for reasons that are not fully understood. This makes it difficult to define the baseline CFRs needed to evaluate treatments in the absence of randomized controls. Here, we investigate whether viremia in EVD patients may be used to evaluate baseline EVD CFRs.
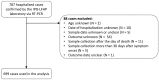
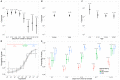
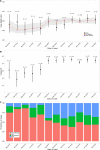
Methods and findings: We analyzed the laboratory and epidemiological records of patients with EVD confirmed by reverse transcription PCR hospitalized in the Conakry area, Guinea, between 1 March 2014 and 28 February 2015. We used viremia and other variables to model the CFR. Data for 699 EVD patients were analyzed. In the week following symptom onset, mean viremia remained stable, and the CFR increased with viremia, V, from 21% (95% CI 16%-27%) for low viremia (V < 104.4 copies/ml) to 53% (95% CI 44%-61%) for intermediate viremia (104.4 ≤ V < 105.2 copies/ml) and 81% (95% CI 75%-87%) for high viremia (V ≥ 105.2 copies/ml). Compared to adults (15-44 y old [y.o.]), the CFR was larger in young children (0-4 y.o.) (odds ratio [OR]: 2.44; 95% CI 1.02-5.86) and older adults (≥ 45 y.o.) (OR: 2.84; 95% CI 1.81-4.46) but lower in children (5-14 y.o.) (OR: 0.46; 95% CI 0.24-0.86). An order of magnitude increase in mean viremia in cases after July 2014 compared to those before coincided with a 14% increase in the CFR. Our findings come from a large hospital-based study in Conakry and may not be generalizable to settings with different case profiles, such as with individuals who never sought care.
Conclusions: Viremia in EVD patients was a strong predictor of death that partly explained variations in CFR in the study population. This study provides baseline CFRs by viremia group, which allow appropriate adjustment when estimating efficacy in treatment studies. In randomized controlled trials, stratifying analysis on viremia groups could reduce sample size requirements by 25%. We hypothesize that monitoring the viremia of hospitalized patients may inform the ability of surveillance systems to detect EVD patients from the different severity strata.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Successful Implementation of a Multicountry Clinical Surveillance and Data Collection System for Ebola Virus Disease in West Africa: Findings and Lessons Learned.Glob Health Sci Pract. 2016 Sep 29;4(3):394-409. doi: 10.9745/GHSP-D-16-00186. Print 2016 Sep 28. Glob Health Sci Pract. 2016. PMID: 27688716 Free PMC article.
-
Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea.PLoS Med. 2016 Mar 1;13(3):e1001967. doi: 10.1371/journal.pmed.1001967. eCollection 2016 Mar. PLoS Med. 2016. PMID: 26930627 Free PMC article. Clinical Trial.
-
Ebola virus disease in children during the 2014-2015 epidemic in Guinea: a nationwide cohort study.Eur J Pediatr. 2017 Jun;176(6):791-796. doi: 10.1007/s00431-017-2914-z. Epub 2017 Apr 25. Eur J Pediatr. 2017. PMID: 28444452
-
Treatment of ebola virus disease.Pharmacotherapy. 2015 Jan;35(1):43-53. doi: 10.1002/phar.1545. Pharmacotherapy. 2015. PMID: 25630412 Review.
-
Case fatality rate for Ebola disease, 1976-2022: A meta-analysis of global data.J Infect Public Health. 2024 Jan;17(1):25-34. doi: 10.1016/j.jiph.2023.10.020. Epub 2023 Oct 27. J Infect Public Health. 2024. PMID: 37992431 Review.
Cited by
-
Vascular dysfunction in hemorrhagic viral fevers: opportunities for organotypic modeling.Biofabrication. 2024 Jun 5;16(3):032008. doi: 10.1088/1758-5090/ad4c0b. Biofabrication. 2024. PMID: 38749416 Free PMC article. Review.
-
Emerging Pathogen Threats in Transfusion Medicine: Improving Safety and Confidence with Pathogen Reduction Technologies.Pathogens. 2023 Jul 5;12(7):911. doi: 10.3390/pathogens12070911. Pathogens. 2023. PMID: 37513758 Free PMC article. Review.
-
Evaluation of viral load in patients with Ebola virus disease in Liberia: a retrospective observational study.Lancet Microbe. 2022 Jul;3(7):e533-e542. doi: 10.1016/S2666-5247(22)00065-9. Epub 2022 May 23. Lancet Microbe. 2022. PMID: 35617976 Free PMC article.
-
Ebola Virus GP Activates Endothelial Cells via Host Cytoskeletal Signaling Factors.Viruses. 2022 Jan 13;14(1):142. doi: 10.3390/v14010142. Viruses. 2022. PMID: 35062347 Free PMC article.
-
Persistence of Ebola virus in semen among Ebola virus disease survivors in Sierra Leone: A cohort study of frequency, duration, and risk factors.PLoS Med. 2021 Feb 10;18(2):e1003273. doi: 10.1371/journal.pmed.1003273. eCollection 2021 Feb. PLoS Med. 2021. PMID: 33566817 Free PMC article.
References
-
- World Health Organization. Ebola situation report. 23 September 2015. Available: http://apps.who.int/iris/bitstream/10665/185279/1/ebolasitrep_23Sept2015.... Accessed 28 September 2015.
-
- Sissoko D, Anglaret X, Malvy D, Folkesson E, Abdoul M, Shepherd S, et al. Favipiravir in patients with Ebola virus disease: early results of the JIKI trial in Guinea [abstract]. Conference on Retroviruses and Opportunistic Infections 2015; 23–26 Feb 2015; Seattle, Washington, US. Available: http://www.croiconference.org/sessions/favipiravir-patients-ebola-virus-.... Accessed 12 May 2015.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


