Designed Amino Acid Feed in Improvement of Production and Quality Targets of a Therapeutic Monoclonal Antibody
- PMID: 26480023
- PMCID: PMC4610691
- DOI: 10.1371/journal.pone.0140597
Designed Amino Acid Feed in Improvement of Production and Quality Targets of a Therapeutic Monoclonal Antibody
Abstract
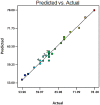
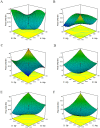
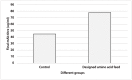
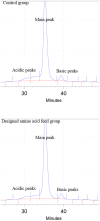
Cell culture feeds optimization is a critical step in process development of pharmaceutical recombinant protein production. Amino acids are the basic supplements of mammalian cell culture feeds with known effect on their growth promotion and productivity. In this study, we reported the implementation of the Plackett-Burman (PB) multifactorial design to screen the effects of amino acids on the growth promotion and productivity of a Chinese hamster ovary DG-44 (CHO-DG44) cell line producing bevacizumab. After this screening, the amino acid combinations were optimized by the response surface methodology (RSM) to determine the most effective concentration in feeds. Through this strategy, the final monoclonal antibody (mAb) titre was enhanced by 70%, compared to the control group. For this particular cell line, aspartic acid, glutamic acid, arginine and glycine had the highest positive effects on the final mAb titre. Simultaneously, the impact of the designed amino acid feed on some critical quality attributes of bevacizumab was examined in the group with highest productivity. The product was analysed for N-glycan profiles, charge variant distribution, and low molecular weight forms. The results showed that the target product quality has been improved using this feeding strategy. It was shown how this strategy could significantly diminish the time and number of experiments in identifying the most effective amino acids and related concentrations in target product enhancement. This model could be successfully applied to other components of culture media and feeds.
Conflict of interest statement
Figures

Similar articles
-
Amino acid and glucose metabolism in fed-batch CHO cell culture affects antibody production and glycosylation.Biotechnol Bioeng. 2015 Mar;112(3):521-35. doi: 10.1002/bit.25450. Epub 2014 Oct 10. Biotechnol Bioeng. 2015. PMID: 25220616
-
Use of a Plackett-Burman statistical design to determine the effect of selected amino acids on monoclonal antibody production in CHO cells.Biotechnol Prog. 2011 Nov-Dec;27(6):1709-17. doi: 10.1002/btpr.674. Epub 2011 Sep 7. Biotechnol Prog. 2011. PMID: 21901863
-
Benchmarking of commercially available CHO cell culture media for antibody production.Appl Microbiol Biotechnol. 2015 Jun;99(11):4645-57. doi: 10.1007/s00253-015-6514-4. Epub 2015 Apr 7. Appl Microbiol Biotechnol. 2015. PMID: 25846330 Free PMC article.
-
Computer-Aided Strategies for Determining the Amino Acid Composition of Medium for Chinese Hamster Ovary Cell-Based Biomanufacturing Platforms.Int J Mol Sci. 2019 Nov 2;20(21):5464. doi: 10.3390/ijms20215464. Int J Mol Sci. 2019. PMID: 31684012 Free PMC article. Review.
-
Applications of small molecules in modulating productivity and product quality of recombinant proteins produced using cell cultures.Biotechnol Adv. 2020 Nov 1;43:107577. doi: 10.1016/j.biotechadv.2020.107577. Epub 2020 Jun 12. Biotechnol Adv. 2020. PMID: 32540474 Review.
Cited by
-
Employing active learning in the optimization of culture medium for mammalian cells.NPJ Syst Biol Appl. 2023 May 30;9(1):20. doi: 10.1038/s41540-023-00284-7. NPJ Syst Biol Appl. 2023. PMID: 37253825 Free PMC article.
-
Applying intensified design of experiments to mammalian cell culture processes.Eng Life Sci. 2021 Nov 24;22(12):784-795. doi: 10.1002/elsc.202100123. eCollection 2022 Dec. Eng Life Sci. 2021. PMID: 36514527 Free PMC article.
-
Clever Experimental Designs: Shortcuts for Better iPSC Differentiation.Cells. 2021 Dec 15;10(12):3540. doi: 10.3390/cells10123540. Cells. 2021. PMID: 34944048 Free PMC article. Review.
-
Serum-Free Medium for Recombinant Protein Expression in Chinese Hamster Ovary Cells.Front Bioeng Biotechnol. 2021 Mar 15;9:646363. doi: 10.3389/fbioe.2021.646363. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33791287 Free PMC article. Review.
-
Digital Twins and Their Role in Model-Assisted Design of Experiments.Adv Biochem Eng Biotechnol. 2021;177:29-61. doi: 10.1007/10_2020_136. Adv Biochem Eng Biotechnol. 2021. PMID: 32797268
References
-
- Gonzalez-Leal IJ, Carrillo-Cocom LM, Ramirez-Medrano A, Lopez-Pacheco F, Bulnes-Abundis D, Webb-Vargas Y, et al. Use of a Plackett-Burman statistical design to determine the effect of selected amino acids on monoclonal antibody production in CHO cells. Biotechnol Prog. 2011;27(6):1709–17. 10.1002/btpr.674 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


