Overexpression of the monocyte chemokine CCL2 in dorsal root ganglion neurons causes a conditioning-like increase in neurite outgrowth and does so via a STAT3 dependent mechanism
- PMID: 26431741
- PMCID: PMC4688065
- DOI: 10.1016/j.expneurol.2015.09.018
Overexpression of the monocyte chemokine CCL2 in dorsal root ganglion neurons causes a conditioning-like increase in neurite outgrowth and does so via a STAT3 dependent mechanism
Abstract
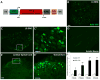
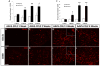
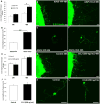
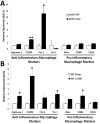
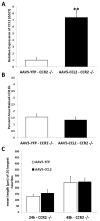
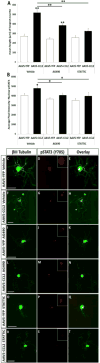
Neuroinflammation plays a critical role in the regeneration of peripheral nerves following axotomy. An injury to the sciatic nerve leads to significant macrophage accumulation in the L5 DRG, an effect not seen when the dorsal root is injured. We recently demonstrated that this accumulation around axotomized cell bodies is necessary for a peripheral conditioning lesion response to occur. Here we asked whether overexpression of the monocyte chemokine CCL2 specifically in DRG neurons of uninjured mice is sufficient to cause macrophage accumulation and to enhance regeneration or whether other injury-derived signals are required. AAV5-EF1α-CCL2 was injected intrathecally, and this injection led to a time-dependent increase in CCL2 mRNA expression and macrophage accumulation in L5 DRG, with a maximal response at 3 weeks post-injection. These changes led to a conditioning-like increase in neurite outgrowth in DRG explant and dissociated cell cultures. This increase in regeneration was dependent upon CCL2 acting through its primary receptor CCR2. When CCL2 was overexpressed in CCR2-/- mice, macrophage accumulation and enhanced regeneration were not observed. To address the mechanism by which CCL2 overexpression enhances regeneration, we tested for elevated expression of regeneration-associated genes in these animals. Surprisingly, we found that CCL2 overexpression led to a selective increase in LIF mRNA and neuronal phosphorylated STAT3 (pSTAT3) in L5 DRGs, with no change in expression seen in other RAGs such as GAP-43. Blockade of STAT3 phosphorylation by each of two different inhibitors prevented the increase in neurite outgrowth. Thus, CCL2 overexpression is sufficient to induce macrophage accumulation in uninjured L5 DRGs and increase the regenerative capacity of DRG neurons via a STAT3-dependent mechanism.
Keywords: CCL2; DRG; MCP-1; Macrophage; Neuroinflammation; Regeneration; STAT3.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: None
Figures
Similar articles
-
CCL2 Mediates Neuron-Macrophage Interactions to Drive Proregenerative Macrophage Activation Following Preconditioning Injury.J Neurosci. 2015 Dec 2;35(48):15934-47. doi: 10.1523/JNEUROSCI.1924-15.2015. J Neurosci. 2015. PMID: 26631474 Free PMC article.
-
The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration.J Neuroinflammation. 2022 Jul 12;19(1):179. doi: 10.1186/s12974-022-02497-9. J Neuroinflammation. 2022. PMID: 35820932 Free PMC article.
-
Macrophage biology in the peripheral nervous system after injury.Prog Neurobiol. 2019 Feb;173:102-121. doi: 10.1016/j.pneurobio.2018.12.001. Epub 2018 Dec 21. Prog Neurobiol. 2019. PMID: 30579784 Free PMC article. Review.
-
A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration.J Neurosci. 2013 Oct 9;33(41):16236-48. doi: 10.1523/JNEUROSCI.3319-12.2013. J Neurosci. 2013. PMID: 24107955 Free PMC article.
-
Unleashing Axonal Regeneration Capacities: Neuronal and Non-neuronal Changes After Injuries to Dorsal Root Ganglion Neuron Central and Peripheral Axonal Branches.Mol Neurobiol. 2024 Jan;61(1):423-433. doi: 10.1007/s12035-023-03590-7. Epub 2023 Aug 24. Mol Neurobiol. 2024. PMID: 37620687 Review.
Cited by
-
Switching Rat Resident Macrophages from M1 to M2 Phenotype by Iba1 Silencing Has Analgesic Effects in SNL-Induced Neuropathic Pain.Int J Mol Sci. 2023 Oct 31;24(21):15831. doi: 10.3390/ijms242115831. Int J Mol Sci. 2023. PMID: 37958812 Free PMC article.
-
Peripheral CCL2 induces inflammatory pain via regulation of Ih currents in small diameter DRG neurons.Front Mol Neurosci. 2023 Oct 4;16:1144614. doi: 10.3389/fnmol.2023.1144614. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37860084 Free PMC article.
-
Transcriptomic analysis of diabetic kidney disease and neuropathy in mouse models of type 1 and type 2 diabetes.Dis Model Mech. 2023 Oct 1;16(10):dmm050080. doi: 10.1242/dmm.050080. Epub 2023 Oct 4. Dis Model Mech. 2023. PMID: 37791586 Free PMC article.
-
Transcriptomic Analysis of the Rat Dorsal Root Ganglion After Fracture.Mol Neurobiol. 2024 Mar;61(3):1467-1478. doi: 10.1007/s12035-023-03637-9. Epub 2023 Sep 19. Mol Neurobiol. 2024. PMID: 37725213
-
Neuronal chemokines: new insights into neuronal communication after injury.Neural Regen Res. 2023 Nov;18(11):2379-2380. doi: 10.4103/1673-5374.371352. Neural Regen Res. 2023. PMID: 37282457 Free PMC article. No abstract available.
References
-
- Alan R, Ezekowitz B, Gordon S. Alterations of surface properties by macrophage activation: expression of receptors for Fc and mannose-terminal glycoproteins and differentiation antigens, Macrophage Activation. Springer. 1984:33–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous


