Complement System Part II: Role in Immunity
- PMID: 26074922
- PMCID: PMC4443744
- DOI: 10.3389/fimmu.2015.00257
Complement System Part II: Role in Immunity
Abstract
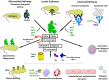
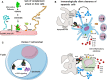
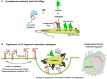
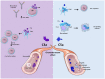
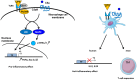
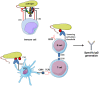
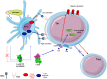
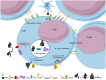
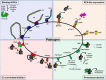
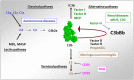
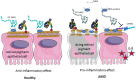
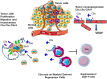
The complement system has been considered for a long time as a simple lytic cascade, aimed to kill bacteria infecting the host organism. Nowadays, this vision has changed and it is well accepted that complement is a complex innate immune surveillance system, playing a key role in host homeostasis, inflammation, and in the defense against pathogens. This review discusses recent advances in the understanding of the role of complement in physiology and pathology. It starts with a description of complement contribution to the normal physiology (homeostasis) of a healthy organism, including the silent clearance of apoptotic cells and maintenance of cell survival. In pathology, complement can be a friend or a foe. It acts as a friend in the defense against pathogens, by inducing opsonization and a direct killing by C5b-9 membrane attack complex and by triggering inflammatory responses with the anaphylatoxins C3a and C5a. Opsonization plays also a major role in the mounting of an adaptive immune response, involving antigen presenting cells, T-, and B-lymphocytes. Nevertheless, it can be also an enemy, when pathogens hijack complement regulators to protect themselves from the immune system. Inadequate complement activation becomes a disease cause, as in atypical hemolytic uremic syndrome, C3 glomerulopathies, and systemic lupus erythematosus. Age-related macular degeneration and cancer will be described as examples showing that complement contributes to a large variety of conditions, far exceeding the classical examples of diseases associated with complement deficiencies. Finally, we discuss complement as a therapeutic target.
Keywords: adaptive immunity; anaphylatoxins; complement and innate immunity; complement in cancer; complement system; complement-related diseases; crosstalk TLR-complement; pathogen strategies for immune evasion.
Figures
Similar articles
-
Complement System Part I - Molecular Mechanisms of Activation and Regulation.Front Immunol. 2015 Jun 2;6:262. doi: 10.3389/fimmu.2015.00262. eCollection 2015. Front Immunol. 2015. PMID: 26082779 Free PMC article. Review.
-
Molecules Great and Small: The Complement System.Clin J Am Soc Nephrol. 2015 Sep 4;10(9):1636-50. doi: 10.2215/CJN.06230614. Epub 2015 Jan 7. Clin J Am Soc Nephrol. 2015. PMID: 25568220 Free PMC article. Review.
-
Self, non-self, and danger: a complementary view.Adv Exp Med Biol. 2006;586:71-94. doi: 10.1007/0-387-34134-X_6. Adv Exp Med Biol. 2006. PMID: 16893066 Review.
-
The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma.Mol Immunol. 2004 Jun;41(2-3):123-31. doi: 10.1016/j.molimm.2004.03.019. Mol Immunol. 2004. PMID: 15159057 Review.
-
[Complement activation and inflammation].Rinsho Byori. 2006 Jul;54(7):744-56. Rinsho Byori. 2006. PMID: 16913666 Review. Japanese.
Cited by
-
Navigating the intricate in-vivo journey of lipid nanoparticles tailored for the targeted delivery of RNA therapeutics: a quality-by-design approach.J Nanobiotechnology. 2024 Nov 14;22(1):710. doi: 10.1186/s12951-024-02972-w. J Nanobiotechnology. 2024. PMID: 39543630 Free PMC article. Review.
-
The plasma proteome reveals markers of recent and repeated stress in free-ranging seals.Conserv Physiol. 2024 Nov 4;12(1):coae075. doi: 10.1093/conphys/coae075. eCollection 2024. Conserv Physiol. 2024. PMID: 39498350 Free PMC article.
-
Complement activation targeted inhibitor C2-FH ameliorates acetaminophen-induced liver injury in mice.World J Hepatol. 2024 Oct 27;16(10):1188-1198. doi: 10.4254/wjh.v16.i10.1188. World J Hepatol. 2024. PMID: 39474574 Free PMC article.
-
Serine protease RAYM_01812 (SspA) inhibits complement-mediated killing and monocyte chemotaxis and contributes to virulence of Riemerella anatipestifer in ducks.Virulence. 2024 Dec;15(1):2421219. doi: 10.1080/21505594.2024.2421219. Epub 2024 Nov 4. Virulence. 2024. PMID: 39450484 Free PMC article.
-
The dysfunction of complement and coagulation in diseases: the implications for the therapeutic interventions.MedComm (2020). 2024 Oct 23;5(11):e785. doi: 10.1002/mco2.785. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39445002 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous


