Protection against live rotavirus challenge in mice induced by parenteral and mucosal delivery of VP6 subunit rotavirus vaccine
- PMID: 26016444
- PMCID: PMC4532722
- DOI: 10.1007/s00705-015-2461-8
Protection against live rotavirus challenge in mice induced by parenteral and mucosal delivery of VP6 subunit rotavirus vaccine
Abstract
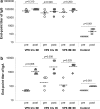
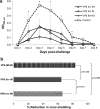
Live oral rotavirus (RV) vaccines are part of routine childhood immunization but are associated with adverse effects, particularly intussusception. We have developed a non-live combined RV - norovirus (NoV) vaccine candidate consisting of human RV inner-capsid rVP6 protein and NoV virus-like particles. To determine the effect of delivery route on induction of VP6-specific protective immunity, BALB/c mice were administered a vaccine containing RV rVP6 intramuscularly, intranasally or a combination of both, and challenged with murine RV. At least 65 % protection against RV shedding was observed regardless of delivery route. The levels of post-challenge serum VP6-specific IgA titers correlated with protection.
Figures
Similar articles
-
Targeting of rotavirus VP6 to DEC-205 induces protection against the infection in mice.Vaccine. 2015 Aug 20;33(35):4228-37. doi: 10.1016/j.vaccine.2015.03.080. Epub 2015 Apr 4. Vaccine. 2015. PMID: 25850020
-
Combination of three virus-derived nanoparticles as a vaccine against enteric pathogens; enterovirus, norovirus and rotavirus.Vaccine. 2019 Dec 3;37(51):7509-7518. doi: 10.1016/j.vaccine.2019.09.072. Epub 2019 Oct 1. Vaccine. 2019. PMID: 31585726
-
Immune responses elicited against rotavirus middle layer protein VP6 inhibit viral replication in vitro and in vivo.Hum Vaccin Immunother. 2014;10(7):2039-47. doi: 10.4161/hv.28858. Hum Vaccin Immunother. 2014. PMID: 25424814 Free PMC article.
-
VP6: A candidate rotavirus vaccine.J Infect Dis. 2010 Sep 1;202 Suppl:S101-7. doi: 10.1086/653556. J Infect Dis. 2010. PMID: 20684688 Review.
-
Human rotavirus virus-like particle vaccines evaluated in a neonatal gnotobiotic pig model of human rotavirus disease.Expert Rev Vaccines. 2013 Feb;12(2):169-81. doi: 10.1586/erv.13.3. Expert Rev Vaccines. 2013. PMID: 23414408 Review.
Cited by
-
Unravelling aggregation propensity of rotavirus A VP6 expressed as E. coli inclusion bodies through in silico prediction.Sci Rep. 2024 Sep 13;14(1):21464. doi: 10.1038/s41598-024-69896-1. Sci Rep. 2024. PMID: 39271700 Free PMC article.
-
Use of live attenuated recombinant Newcastle disease virus carrying avian paramyxovirus 2 HN and F protein genes to enhance immune responses against species A rotavirus VP6 protein.Vet Res. 2024 Feb 5;55(1):16. doi: 10.1186/s13567-024-01271-4. Vet Res. 2024. PMID: 38317245 Free PMC article.
-
Generation of Recombinant Rotaviruses Expressing Human Norovirus Capsid Proteins.J Virol. 2022 Nov 23;96(22):e0126222. doi: 10.1128/jvi.01262-22. Epub 2022 Oct 31. J Virol. 2022. PMID: 36314817 Free PMC article.
-
Rotavirus VP6: involvement in immunogenicity, adjuvant activity, and use as a vector for heterologous peptides, drug delivery, and production of nano-biomaterials.Arch Virol. 2022 Apr;167(4):1013-1023. doi: 10.1007/s00705-022-05407-9. Epub 2022 Mar 15. Arch Virol. 2022. PMID: 35292854 Free PMC article. Review.
-
Rotavirus vaccines: progress and new developments.Expert Opin Biol Ther. 2022 Mar;22(3):423-432. doi: 10.1080/14712598.2021.1977279. Epub 2021 Sep 10. Expert Opin Biol Ther. 2022. PMID: 34482790 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous


