Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis
- PMID: 25975452
- PMCID: PMC4580232
- DOI: 10.1016/S0140-6736(14)61704-9
Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis
Abstract
Background: Serious infections are a major concern for patients considering treatments for rheumatoid arthritis. Evidence is inconsistent as to whether biological drugs are associated with an increased risk of serious infection compared with traditional disease-modifying antirheumatic drugs (DMARDs). We did a systematic review and meta-analysis of serious infections in patients treated with biological drugs compared with those treated with traditional DMARDs.
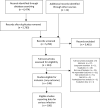
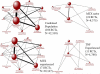
Methods: We did a systematic literature search with Medline, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov from their inception to Feb 11, 2014. Search terms included "biologics", "rheumatoid arthritis" and their synonyms. Trials were eligible for inclusion if they included any of the approved biological drugs and reported serious infections. We assessed the risk of bias with the Cochrane Risk of Bias Tool. We did a Bayesian network meta-analysis of published trials using a binomial likelihood model to assess the risk of serious infections in patients with rheumatoid arthritis who were treated with biological drugs, compared with those treated with traditional DMARDs. The odds ratio (OR) of serious infection was the primary measure of treatment effect and calculated 95% credible intervals using Markov Chain Monte Carlo methods.
Findings: The systematic review identified 106 trials that reported serious infections and included patients with rheumatoid arthritis who received biological drugs. Compared with traditional DMARDs, standard-dose biological drugs (OR 1.31, 95% credible interval [CrI] 1.09-1.58) and high-dose biological drugs (1.90, 1.50-2.39) were associated with an increased risk of serious infections, although low-dose biological drugs (0.93, 0.65-1.33) were not. The risk was lower in patients who were methotrexate naive compared with traditional DMARD-experienced or anti-tumour necrosis factor biological drug-experienced patients. The absolute increase in the number of serious infections per 1000 patients treated each year ranged from six for standard-dose biological drugs to 55 for combination biological therapy, compared with traditional DMARDs.
Interpretation: Standard-dose and high-dose biological drugs (with or without traditional DMARDs) are associated with an increase in serious infections in rheumatoid arthritis compared with traditional DMARDs, although low-dose biological drugs are not. Clinicians should discuss the balance between benefit and harm with the individual patient before starting biological treatment for rheumatoid arthritis.
Funding: Rheumatology Division at the University of Alabama at Birmingham.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Rheumatoid arthritis: biological drugs and risk of infection.Lancet. 2015 Jul 18;386(9990):224-5. doi: 10.1016/S0140-6736(14)61907-3. Epub 2015 May 11. Lancet. 2015. PMID: 25975453 No abstract available.
-
ACP Journal Club. Review: In RA, standard- and high-dose biologic drugs increase serious infection compared with traditional DMARDs.Ann Intern Med. 2015 Oct 20;163(8):JC5. doi: 10.7326/ACPJC-2015-163-8-005. Ann Intern Med. 2015. PMID: 26502142 No abstract available.
-
[Serious infections in patients with rheumatoid arthritis].Internist (Berl). 2016 Mar;57(3):298-300. doi: 10.1007/s00108-015-0012-8. Internist (Berl). 2016. PMID: 26838367 German. No abstract available.
Similar articles
-
Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials.JAMA. 2006 May 17;295(19):2275-85. doi: 10.1001/jama.295.19.2275. JAMA. 2006. PMID: 16705109 Review.
-
ACP Journal Club. Review: In RA, standard- and high-dose biologic drugs increase serious infection compared with traditional DMARDs.Ann Intern Med. 2015 Oct 20;163(8):JC5. doi: 10.7326/ACPJC-2015-163-8-005. Ann Intern Med. 2015. PMID: 26502142 No abstract available.
-
A meta-analysis of the efficacy and toxicity of combining disease-modifying anti-rheumatic drugs in rheumatoid arthritis based on patient withdrawal.Rheumatology (Oxford). 2005 Nov;44(11):1414-21. doi: 10.1093/rheumatology/kei031. Epub 2005 Jul 19. Rheumatology (Oxford). 2005. PMID: 16030080 Review.
-
Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis.Ann Intern Med. 2008 Jan 15;148(2):124-34. doi: 10.7326/0003-4819-148-2-200801150-00192. Epub 2007 Nov 19. Ann Intern Med. 2008. PMID: 18025440 Review.
-
Glucocorticoid use in rheumatoid arthritis patients and the onset of pneumonia: a systematic review and meta-analysis.J Osteopath Med. 2023 Jan 25;123(4):179-186. doi: 10.1515/jom-2022-0177. eCollection 2023 Apr 1. J Osteopath Med. 2023. PMID: 36691851 Review.
Cited by
-
Rheumatoid arthritis and COVID-19 outcomes: a systematic review and Meta-analysis.BMC Rheumatol. 2024 Nov 12;8(1):61. doi: 10.1186/s41927-024-00431-5. BMC Rheumatol. 2024. PMID: 39529202 Free PMC article.
-
Effect of intravitreal injection of anti-interleukin (IL)-6 antibody in experimental autoimmune uveitis in mice.J Ophthalmic Inflamm Infect. 2024 Nov 4;14(1):57. doi: 10.1186/s12348-024-00441-x. J Ophthalmic Inflamm Infect. 2024. PMID: 39497001 Free PMC article.
-
Management strategies in rheumatoid arthritis.Nat Rev Rheumatol. 2024 Dec;20(12):760-769. doi: 10.1038/s41584-024-01169-7. Epub 2024 Oct 24. Nat Rev Rheumatol. 2024. PMID: 39448800 Review.
-
Incidence of serious respiratory tract infections and associated characteristics in a population exposed to immunosuppressive therapies: a register-based population study.BMC Infect Dis. 2024 Oct 21;24(1):1184. doi: 10.1186/s12879-024-10039-2. BMC Infect Dis. 2024. PMID: 39434000 Free PMC article.
-
Turning the Tide against Herpes Zoster in Rheumatoid Arthritis Patients Treated with JAK Inhibitors.J Clin Med. 2024 Jul 29;13(15):4423. doi: 10.3390/jcm13154423. J Clin Med. 2024. PMID: 39124690 Free PMC article.
References
-
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–108. - PubMed
-
- Tugwell P, Singh JA, Wells GA. Biologicals for rheumatoid arthritis. BMJ. 2011;343:d4027. - PubMed
-
- Ioannidis JP, Karassa FB, Druyts E, Thorlund K, Mills EJ. Biologic agents in rheumatology: unmet issues after 200 trials and $200 billion sales. Nature reviews Rheumatology. 2013;9(11):665–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical


