Dimethyl fumarate for multiple sclerosis
- PMID: 25900414
- PMCID: PMC10663978
- DOI: 10.1002/14651858.CD011076.pub2
Dimethyl fumarate for multiple sclerosis
Abstract
Background: Multiple sclerosis (MS) often leads to severe neurological disability and a serious decline in quality of life. The ideal target of disease-modifying therapy for MS is to prevent disability worsening and improve quality of life. Dimethyl fumarate is considered to have an immunomodulatory activity and neuroprotective effect. It has been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency as a first-line therapy for adult patients with relapsing-remitting MS (RMSS).
Objectives: To assess the benefit and safety of dimethyl fumarate as monotherapy or combination therapy versus placebo or other approved disease-modifying drugs (interferon beta, glatiramer acetate, natalizumab, mitoxantrone, fingolimod, teriflunomide, alemtuzumab) for patients with MS.
Search methods: The Trials Search Co-ordinator searched the Trials Specialised Register of the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group (4 June 2014). We checked reference lists of published reviews and retrieved articles and searched reports (2004 to June 2014) from the MS societies in Europe and America. We also communicated with investigators participating in trials of dimethyl fumarate and the Biogen Idec Medical Information.
Selection criteria: We included randomised, controlled, parallel-group clinical trials (RCTs) with a length of follow-up equal to or greater than one year evaluating dimethyl fumarate, as monotherapy or combination therapy, versus placebo or other approved disease-modifying drugs for patients with MS without restrictions regarding dosage, administration frequency and duration of treatment.
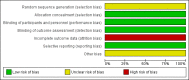
Data collection and analysis: We used the standard methodological procedures of The Cochrane Collaboration. Two review authors independently assessed trial quality and extracted data. Disagreements were discussed and resolved by consensus among the review authors. We contacted the principal investigators of included studies for additional data or confirmation of data.
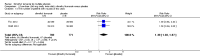
Main results: Two RCTs were included, involving 2667 adult patients with RRMS to evaluate the efficacy and safety of two dosages of dimethyl fumarate (240 mg orally three times daily or twice daily) by direct comparison with placebo for two years. Among them, a subsample of 1221 (45.8%) patients were selected to participate in MRI evaluations by each study site with MRI capabilities itself. No powered head-to-head study with an active treatment comparator has been found. Meta-analyses showed that dimethyl fumarate both three times daily and twice daily reduced the number of patients with a relapse (risk ratio (RR) 0.57, 95% confidence interval (CI) 0.50 to 0.66, P < 0.00001 and 0.64, 95% CI 0.54 to 0.77, P < 0.00001, respectively) or disability worsening (RR 0.70, 95% CI 0.57 to 0.87, P = 0.0009 and 0.65, 95% CI 0.53 to 0.81, P = 0.0001, respectively) over two years, compared to placebo. The treatment effects were decreased in the likely-case scenario analyses taking the effect of dropouts into consideration. Both dosages also reduced the annualised relapse rate. Data of active lesions on MRI scans were not combined because there was a high risk of selection bias for MRI outcomes and imprecision of MRI data in both studies, as well as an obvious heterogeneity between the studies. In terms of safety profile, both dosages increased the risk for adverse events and the risk for drug discontinuation due to adverse events. The most common adverse events included flushing and gastrointestinal events (upper abdominal pain, nausea and diarrhoea). Uncommon adverse events included lymphopenia and leukopenia, but they were more likely to happen with dimethyl fumarate than with placebo (high dosage: RR 5.25, 95% CI 2.20 to 12.51, P = 0.0002 and 5.23, 95% CI 2.47 to 11.07, P < 0.0001, respectively; low dosage: RR 5.69, 95% CI 2.40 to 13.46, P < 0.0001 and 6.53, 95% CI 3.13 to 13.64, P < 0.00001, respectively). Both studies had a high attrition bias resulting from the unbalanced reasons for dropouts among groups. Quality of evidence for relapse outcome was moderate, but for disability worsening was low.
Authors' conclusions: There is moderate-quality evidence to support that dimethyl fumarate at a dose of 240 mg orally three times daily or twice daily reduces both the number of patients with a relapse and the annualised relapse rate over two years of treatment in comparison with placebo. However, the quality of the evidence to support the benefit in reducing the number of patients with disability worsening is low. There is no high-quality data available to evaluate the benefit on MRI outcomes. The common adverse effects such as flushing and gastrointestinal events are mild-to-moderate for most patients. Lymphopenia and leukopenia are uncommon adverse events but significantly associated with dimethyl fumarate. Both dosages of dimethyl fumarate have similar benefit and safety profile, which supports the option of low-dose administration. New studies of high quality and long-term follow-up are needed to evaluate the benefit of dimethyl fumarate on prevention of disability worsening and to observe the long-term adverse effects including progressive multifocal leukoencephalopathy.
Conflict of interest statement
HD ‐ none
XZ ‐ none
ZF ‐ none
SFL ‐ none
GKF‐ none
DS ‐ none
Figures
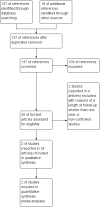










































Update of
- doi: 10.1002/14651858.CD011076
Similar articles
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2016 Mar 22;3(3):CD009882. doi: 10.1002/14651858.CD009882.pub3. Cochrane Database Syst Rev. 2016. PMID: 27003123 Free PMC article. Review.
-
Rituximab for relapsing-remitting multiple sclerosis.Cochrane Database Syst Rev. 2013 Dec 6;(12):CD009130. doi: 10.1002/14651858.CD009130.pub3. Cochrane Database Syst Rev. 2013. PMID: 24310855 Review.
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article. Review.
-
Siponimod for multiple sclerosis.Cochrane Database Syst Rev. 2021 Nov 16;11(11):CD013647. doi: 10.1002/14651858.CD013647.pub2. Cochrane Database Syst Rev. 2021. PMID: 34783010 Free PMC article. Review.
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2015 Sep 18;2015(9):CD011381. doi: 10.1002/14651858.CD011381.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD011381. doi: 10.1002/14651858.CD011381.pub3 PMID: 26384035 Free PMC article. Updated. Review.
Cited by
-
Small molecule α-methylene-γ-butyrolactone, an evolutionarily conserved moiety in sesquiterpene lactones, ameliorates arthritic phenotype via interference DNA binding activity of NF-κB.Acta Pharm Sin B. 2024 Aug;14(8):3561-3575. doi: 10.1016/j.apsb.2024.04.004. Epub 2024 Apr 8. Acta Pharm Sin B. 2024. PMID: 39220880 Free PMC article.
-
Potential use of antioxidants for the treatment of chronic inflammatory diseases.Front Pharmacol. 2024 May 16;15:1378335. doi: 10.3389/fphar.2024.1378335. eCollection 2024. Front Pharmacol. 2024. PMID: 38818374 Free PMC article. Review.
-
Dimethyl fumarate alleviates allergic asthma by strengthening the Nrf2 signaling pathway in regulatory T cells.Front Immunol. 2024 Apr 22;15:1375340. doi: 10.3389/fimmu.2024.1375340. eCollection 2024. Front Immunol. 2024. PMID: 38711519 Free PMC article.
-
A comparison of clinical, utilization, and cost outcomes between oral treatments for multiple sclerosis.J Manag Care Spec Pharm. 2024 Feb 3;30(2):129-140. doi: 10.18553/jmcp.2024.30.2.129. J Manag Care Spec Pharm. 2024. PMID: 38308623 Free PMC article.
-
Dimethyl fumarate in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.Nat Commun. 2024 Jan 31;15(1):924. doi: 10.1038/s41467-023-43644-x. Nat Commun. 2024. PMID: 38296965 Free PMC article. Clinical Trial.
References
References to studies included in this review
Fox 2012 {published data only}
-
- Fox R, Miller D, Phillips JT, Kita M, Hutchinson M, Havrdova E, et al. Clinical efficacy of BG‐12 in relapsing‐remitting multiple sclerosis (RRMS): data from the phase 3 CONFIRM study. Meeting abstracts of the The American Academy of Neurology's 64th AAN Annual Meeting 2012.
-
- Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo‐controlled phase 3 study of oral BG‐12 or glatiramer in multiple sclerosis. New England Journal of Medicine 2012;367(12):1087‐97. - PubMed
-
- Havrdova E, Miller D, Fox RJ, Phillips JT, Kita M, Hutchinson M, et al. Clinical and neuroimaging outcomes with BG‐12 treatment in CONFIRM (comparator and an oral fumarate in relapsing‐remitting multiple sclerosis) a multicenter, randomized, placebo‐controlled, phase‐3 study. European Journal of Neurology 2012;19:87.
-
- Havrdova E, Miller D, Fox RJ, Phillips JT, Kita M, Hutchinson M, et al. Clinical outcomes with BG‐12 treatment in CONFIRM (Comparator and an oral fumarate in relapsing‐ remitting multiple sclerosis) a multicentre, randomised, placebo‐controlled, phase 3 study. Journal of Neurology 2012;259(1 Suppl):36.
-
- Hutchinson M, Fox RJ, Miller D, Phillips JT, Kita M, Havrdova E, et al. Effect of BG‐12 in subgroups of patients with relapsing‐remitting multiple sclerosis: findings from the CONFIRM (comparator and an oral fumarate in relapsing‐remitting multiple sclerosis) study. European Journal of Neurology 2012;19:355.
Gold 2012 {published data only}
-
- Agarwal S, Kappos L, Gold R, Arnold D, Bar‐Or A, Giovannoni G, et al. Effects of BG‐12 on quality of life in patients with relapsing‐remitting multiple sclerosis: findings from the DEFINE study. Meeting abstracts of the The American Academy of Neurology's 64th AAN Annual Meeting 2012.
-
- Arnold D, Gold R, Bar‐Or A, Kappos L, Giovannoni G, Selmaj K, et al. Efficacy on MRI endpoints of BG‐12, an oral therapy, in relapsing‐remitting multiple sclerosis: data from the phase 3 DEFINE trial. Multiple Sclerosis 2011;17(10 Suppl):369‐70.
-
- Arnold D, Gold R, Kappos L, Bar‐Or A, Giovannoni G, Selmaj K, et al. Effect of BG‐12 on brain atrophy and lesions volume: MRI results from the define study during first and second year of treatment. Meeting abstracts of the The American Academy of Neurology's 64th AAN Annual Meeting.
-
- Arnold D, Gold R, Kappos L, Bar‐Or A, Giovannoni G, Selmaj K, et al. Effects of BG‐12 on magnetization transfer ratio in whole brain and normal‐appearing brain tissue: findings from the DEFINE study. Meeting abstracts of the The American Academy of Neurology's 64th AAN Annual Meeting 2012.
-
- Bar‐Or A, Gold R, Kappos L, Arnold DL, Giovannoni G, Selmaj K, et al. Clinical efficacy of BG‐12 (dimethyl fumarate) in patients with relapsing‐remitting multiple sclerosis: subgroup analyses of the DEFINE study. Journal of Neurology 2013;260(9):2297‐305. - PubMed
References to studies excluded from this review
Kappos 2008 {published data only}
-
- Kappos L, Gold R, Miller DH, MacManus DG, Havrdova E, Limmroth V, et al. Effect of BG‐12 on contrast‐enhanced lesions in patients with relapsing‐remitting multiple sclerosis: subgroup analyses from the phase 2b study. Multiple Sclerosis 2012;18(3):314‐21. - PubMed
-
- Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, et al. Efficacy and safety of oral fumarate in patients with relapsing‐remitting multiple sclerosis: a multicentre, randomised, double‐blind, placebo‐controlled phase IIb study. Lancet 2008;372(9648):1463‐72. - PubMed
-
- MacManus DG, Miller DH, Kappos L, Gold R, Havrdova E, Limmroth V, et al. BG‐12 reduces evolution of new enhancing lesions to T1‐hypointense lesions in patients with multiple sclerosis. Journal of Neurology 2011;258(3):449‐56. - PubMed
Schimrigk 2006 {published data only}
-
- Schimrigk S, Brune N, Hellwig K, Lukas C, Bellenberg B, Rieks M, et al. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open‐label, baseline‐controlled pilot study. European Journal of Neurology 2006;13(6):604‐10. - PubMed
Additional references
Albrecht 2012
Cella 1996
-
- Cella DF, Dineen K, Arnason B, Reder A, Webster KA, Karabatsos G, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology 1996;47(1):129‐39. - PubMed
Confavreux 2006
-
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain 2006;129(3):606‐16. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Ermis 2013
-
- Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. New England Journal of Medicine 2013;368(17):1657‐8. - PubMed
Filippini 2013
Fischer 1999
-
- Fischer JS, Rocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Multiple Sclerosis 1999;5(4):251‐9. - PubMed
Ghoreschi 2011
Gilgun‐Sherki 2004
-
- Gilgun‐Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. Journal of Neurology 2004;251(3):261‐8. - PubMed
GRADEpro 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Brozek J, Oxman A, Schünemann H, 2008.
Greenland 1985
-
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41(1):55‐68. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hutchinson 2014
-
- Hutchinson M, Fox RJ, Havrdova E, Kurukulasuriya NC, Sarda SP, Agarwal S, et al. Efficacy and safety of BG‐12 (dimethyl fumarate) and other disease‐modifying therapies for the treatment of relapsing‐remitting multiple sclerosis: a systematic review and mixed treatment comparison. Current Medical Research Opinion 2014; Vol. 30, issue 4:613‐27. - PubMed
Johnson 2010
Karampampa 2012a
-
- Karampampa K, Gustavsson A, Miltenburger C, Eckert B. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Multiple Sclerosis 2012;18(2 Suppl):7‐15. - PubMed
Karampampa 2012c
-
- Karampampa K, Gustavsson A, Miltenburger C, Kindundu CM, Selchen DH. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis: the costs and utilities of MS patients in Canada. Journal of Population Therapeutics and Clinical Pharmacology 2012;19(1):11‐25. - PubMed
Karampampa 2013b
-
- Karampampa K, Gustavsson A, Munster ET, Hupperts RM, Sanders EA, Mostert J, et al. Treatment experience, burden, and unmet needs (TRIBUNE) in Multiple Sclerosis study: the costs and utilities of MS patients in The Netherlands. Journal of Medical Economics 2013;16(7):939‐50. - PubMed
Kawalec 2014
Kurtzke 1983
-
- Kurtzke JF. Rating neurological impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33:1444‐52. - PubMed
Lee 2008
-
- Lee DH, Linker RA, Gold R. Spotlight on fumarates. International MS Journal 2008;15(1):12‐8. - PubMed
Lee 2012
Linker 2011
-
- Linker RA, Lee DH, Ryan S, Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134(3):678‐92. - PubMed
Litjens 2004
Lublin 1996
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907‐11. - PubMed
Lukashev 2007
-
- Lukashev M, Zeng W, Goelz S, Lee D, Linker R, Gold R, et al. Activation of Nrf2 and modulation of disease progression in EAE models by BG00012 (dimethyl fumarate) suggests a novel mechanism of action combining anti‐inflammatory and neuroprotective modalities. Multiple Sclerosis 2007;13(Suppl 2):149.
Mantel 1959
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. - PubMed
McDonald 2001
-
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. - PubMed
National MS Society 2013
-
- National MS Society. Tecfidera. http://www.nationalmssociety.org/Treating‐MS/Medications/Tecfidera™ (accessed 2 September 2014).
Oleen‐Burkey 2012
-
- Oleen‐Burkey M, Castelli‐Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse: the patient's perspective. Patient 2012;5(1):57‐69. - PubMed
Peng 2012
-
- Peng H, Guerau‐de‐Arellano M, Mehta VB, Yang Y, Huss DJ, Papenfuss TL, et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF‐κB) and extracellular signal‐regulated kinase 1 and 2 (ERK1/2) and mitogen stress‐activated kinase 1 (MSK1) signaling. Journal of Biological Chemistry 2012;287(33):28017‐26. - PMC - PubMed
Polman 2005
-
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of Neurology 2005;58(6):840‐6. - PubMed
Polman 2011
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(8):227‐31. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scannevin 2012
-
- Scannevin RH, Chollate S, Jung MY, Shackett M, Patel H, Bista P, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid‐derived 2)‐like 2 pathway. Journal of Pharmacology and Experimental Therapeutics 2012;341(1):274‐84. - PubMed
Schilling 2006
Shirani 2012
-
- Shirani A, Zhao Y, Karim ME, Evans C, Kingwell E, Kop ML, et al. Association between use of interferon beta and progression of disability in patients with relapsing‐remitting multiple sclerosis. JAMA 2012;308(3):247‐56. - PubMed
Sweetser 2013
-
- Sweetser MT, Dawson KT, Bozic C. Manufacturer's response to case reports of PML. New England Journal of Medicine 2013;368(17):1659‐61. - PubMed
Tullman 2004
-
- Tullman MJ, Oshinsky RJ, Lublin FD, Cutter GR. Clinical characteristics of progressive relapsing multiple sclerosis. Multiple Sclerosis 2004;10(4):451‐4. - PubMed
U.S. Food and Drug Administration 2014
-
- U.S. Food, Drug Administration. FDA Drug Safety Communication: FDA warns about case of rare brain infection PML with MS drug Tecfidera (dimethyl fumarate). http://www.fda.gov/Drugs/DrugSafety/ucm424625.htm (accessed 25 November 2014).
van Oosten 2013
-
- Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. New England Journal of Medicine 2013;368(17):1658‐9. - PubMed
Vickrey 1995
-
- Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health‐related quality of life measure for multiple sclerosis. Quality of Life Research 1995;4(3):187‐206. - PubMed
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. - PubMed
Wilms 2010
-
- Wilms H, Sievers J, Rickert U, Rostami‐Yazdi M, Mrowietz U, Lucius R. Dimethyl fumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL‐1beta, TNF‐alpha and IL‐6 in an in‐vitro model of brain inflammation. Journal of Neuroinflammation 2010;7:30. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources


