The trace amine-associated receptor 1 modulates methamphetamine's neurochemical and behavioral effects
- PMID: 25762894
- PMCID: PMC4327507
- DOI: 10.3389/fnins.2015.00039
The trace amine-associated receptor 1 modulates methamphetamine's neurochemical and behavioral effects
Abstract
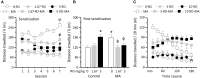
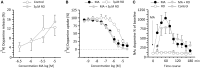
The newly discovered trace amine-associated receptor 1 (TAAR1) has the ability to regulate both dopamine function and psychostimulant action. Here, we tested in rats the ability of RO5203648, a selective TAAR1 partial agonist, to modulate the physiological and behavioral effects of methamphetamine (METH). In experiment 1, RO5203468 dose- and time-dependently altered METH-induced locomotor activity, manifested as an early attenuation followed by a late potentiation of METH's stimulating effects. In experiment 2, rats received a 14-day treatment regimen during which RO5203648 was co-administered with METH. RO5203648 dose-dependently attenuated METH-stimulated hyperactivity, with the effects becoming more apparent as the treatments progressed. After chronic exposure and 3-day withdrawal, rats were tested for locomotor sensitization. RO5203648 administration during the sensitizing phase prevented the development of METH sensitization. However, RO5203648, at the high dose, cross-sensitized with METH. In experiment 3, RO5203648 dose-dependently blocked METH self-administration without affecting operant responding maintained by sucrose, and exhibited lack of reinforcing efficacy when tested as a METH's substitute. Neurochemical data showed that RO5203648 did not affect METH-mediated DA efflux and uptake inhibition in striatal synaptosomes. In vivo, however, RO5203648 was able to transiently inhibit METH-induced accumulation of extracellular DA levels in the nucleus accumbens. Taken together, these data highlight the significant potential of TAAR1 to modulate METH's neurochemical and behavioral effects.
Keywords: methamphetamine; microdialysis; self-administration; sensitization; synaptosomes; trace amine-associated receptor.
Figures

Similar articles
-
Pharmacological Treatments for Methamphetamine Use Disorder: Current Status and Future Targets.Subst Abuse Rehabil. 2024 Aug 30;15:125-161. doi: 10.2147/SAR.S431273. eCollection 2024. Subst Abuse Rehabil. 2024. PMID: 39228432 Free PMC article. Review.
-
A partial trace amine-associated receptor 1 agonist exhibits properties consistent with a methamphetamine substitution treatment.Addict Biol. 2017 Sep;22(5):1246-1256. doi: 10.1111/adb.12410. Epub 2016 May 19. Addict Biol. 2017. PMID: 27193165
-
Selective activation of the trace amine-associated receptor 1 decreases cocaine's reinforcing efficacy and prevents cocaine-induced changes in brain reward thresholds.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Dec 3;63:70-5. doi: 10.1016/j.pnpbp.2015.05.014. Epub 2015 Jun 3. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 26048337
-
Methamphetamine-induced dopamine terminal deficits in the nucleus accumbens are exacerbated by reward-associated cues and attenuated by CB1 receptor antagonism.Neuropharmacology. 2012 Jun;62(7):2192-201. doi: 10.1016/j.neuropharm.2012.01.013. Epub 2012 Jan 25. Neuropharmacology. 2012. PMID: 22306525 Free PMC article.
-
Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum.Life Sci. 2003 Jun 27;73(6):727-39. doi: 10.1016/s0024-3205(03)00393-x. Life Sci. 2003. PMID: 12801594 Review.
Cited by
-
Pharmacological Treatments for Methamphetamine Use Disorder: Current Status and Future Targets.Subst Abuse Rehabil. 2024 Aug 30;15:125-161. doi: 10.2147/SAR.S431273. eCollection 2024. Subst Abuse Rehabil. 2024. PMID: 39228432 Free PMC article. Review.
-
Recognition of methamphetamine and other amines by trace amine receptor TAAR1.Nature. 2023 Dec;624(7992):663-671. doi: 10.1038/s41586-023-06775-1. Epub 2023 Nov 7. Nature. 2023. PMID: 37935377
-
Robust aversive effects of trace amine-associated receptor 1 activation in mice.Neuropsychopharmacology. 2023 Sep;48(10):1446-1454. doi: 10.1038/s41386-023-01578-4. Epub 2023 Apr 13. Neuropsychopharmacology. 2023. PMID: 37055488 Free PMC article.
-
Dopamine, Immunity, and Disease.Pharmacol Rev. 2023 Jan;75(1):62-158. doi: 10.1124/pharmrev.122.000618. Epub 2022 Dec 8. Pharmacol Rev. 2023. PMID: 36757901 Free PMC article. Review.
-
Trace amine-associated receptor 1 and drug abuse.Adv Pharmacol. 2022;93:373-401. doi: 10.1016/bs.apha.2021.10.005. Epub 2021 Nov 11. Adv Pharmacol. 2022. PMID: 35341572 Free PMC article.
References
-
- Bradaia A., Trube G., Stalder H., Norcross R. D., Ozmen L., Wettstein J. G., et al. . (2009). The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc. Natl. Acad. Sci. U.S.A. 106, 20081–20086. 10.1073/pnas.0906522106 - DOI - PMC - PubMed
-
- Bunzow J. R., Sonders M. S., Arttamangkul S., Harrison L. M., Zhang G., Quigley D. I., et al. . (2001). Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 60, 1181–1188. 10.1124/mol.60.6.1181 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources


