The ins and outs of MHC class II-mediated antigen processing and presentation
- PMID: 25720354
- PMCID: PMC6314495
- DOI: 10.1038/nri3818
The ins and outs of MHC class II-mediated antigen processing and presentation
Abstract
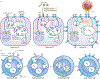
Antigenic peptide-loaded MHC class II molecules (peptide-MHC class II) are constitutively expressed on the surface of professional antigen-presenting cells (APCs), including dendritic cells, B cells, macrophages and thymic epithelial cells, and are presented to antigen-specific CD4(+) T cells. The mechanisms of antigen uptake, the nature of the antigen processing compartments and the lifetime of cell surface peptide-MHC class II complexes can vary depending on the type of APC. It is likely that these differences are important for the function of each distinct APC subset in the generation of effective adaptive immune responses. In this Review, we describe our current knowledge of the mechanisms of uptake and processing of antigens, the intracellular formation of peptide-MHC class II complexes, the intracellular trafficking of peptide-MHC class II complexes to the APC plasma membrane and their ultimate degradation.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures

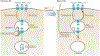
Similar articles
-
Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition.J Immunol. 1999 Nov 15;163(10):5201-10. J Immunol. 1999. PMID: 10553040
-
A central role for HSC70 in regulating antigen trafficking and MHC class II presentation.Mol Immunol. 2015 Dec;68(2 Pt A):85-8. doi: 10.1016/j.molimm.2015.04.007. Epub 2015 May 4. Mol Immunol. 2015. PMID: 25953005 Free PMC article. Review.
-
Mhc-guided processing: binding of large antigen fragments.Nat Rev Immunol. 2003 Aug;3(8):621-9. doi: 10.1038/nri1149. Nat Rev Immunol. 2003. PMID: 12974477 Review.
-
Antigen-independent acquisition of MHC class II molecules by human T lymphocytes.Int Immunol. 2004 Oct;16(10):1523-33. doi: 10.1093/intimm/dxh154. Epub 2004 Sep 6. Int Immunol. 2004. PMID: 15351785 Free PMC article.
-
Processing and MHC class II presentation of exogenous soluble antigen involving a proteasome-dependent cytosolic pathway in CD40-activated B cells.Eur J Haematol. 2016 Aug;97(2):166-74. doi: 10.1111/ejh.12699. Epub 2015 Dec 10. Eur J Haematol. 2016. PMID: 26561366
Cited by
-
ZBTB48 is a priming factor regulating B-cell-specific CIITA expression.EMBO J. 2024 Nov 19. doi: 10.1038/s44318-024-00306-y. Online ahead of print. EMBO J. 2024. PMID: 39562739
-
Hypoxia-induced PD-L1 expression and modulation of muscle stem cell allograft rejection.Front Pharmacol. 2024 Nov 1;15:1471563. doi: 10.3389/fphar.2024.1471563. eCollection 2024. Front Pharmacol. 2024. PMID: 39555101 Free PMC article.
-
Therapeutic potential of natural coumarins in autoimmune diseases with underlying mechanisms.Front Immunol. 2024 Oct 31;15:1432846. doi: 10.3389/fimmu.2024.1432846. eCollection 2024. Front Immunol. 2024. PMID: 39544933 Free PMC article. Review.
-
Rapid parallel reconstruction and specificity screening of hundreds of T cell receptors.Nat Protoc. 2024 Nov 8. doi: 10.1038/s41596-024-01061-4. Online ahead of print. Nat Protoc. 2024. PMID: 39516267 Review.
-
Border-Associated Macrophages: From Embryogenesis to Immune Regulation.CNS Neurosci Ther. 2024 Nov;30(11):e70105. doi: 10.1111/cns.70105. CNS Neurosci Ther. 2024. PMID: 39496482 Free PMC article. Review.
References
-
- Banchereau J et al. Immunobiology of dendritic cells. Annu. Rev. Immunol 18, 767–811 (2000). - PubMed
-
- Harwood NE & Batista FD Early events in B cell activation. Annu. Rev. Immunol 28, 185–210 (2010). - PubMed
-
- Trombetta ES & Mellman I Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol 23, 975–1028 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous


