Type I interferons in infectious disease
- PMID: 25614319
- PMCID: PMC7162685
- DOI: 10.1038/nri3787
Type I interferons in infectious disease
Abstract
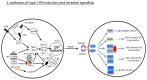
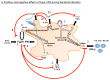
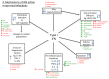
Type I interferons (IFNs) have diverse effects on innate and adaptive immune cells during infection with viruses, bacteria, parasites and fungi, directly and/or indirectly through the induction of other mediators. Type I IFNs are important for host defence against viruses. However, recently, they have been shown to cause immunopathology in some acute viral infections, such as influenza virus infection. Conversely, they can lead to immunosuppression during chronic viral infections, such as lymphocytic choriomeningitis virus infection. During bacterial infections, low levels of type I IFNs may be required at an early stage, to initiate cell-mediated immune responses. High concentrations of type I IFNs may block B cell responses or lead to the production of immunosuppressive molecules, and such concentrations also reduce the responsiveness of macrophages to activation by IFNγ, as has been shown for infections with Listeria monocytogenes and Mycobacterium tuberculosis. Recent studies in experimental models of tuberculosis have demonstrated that prostaglandin E2 and interleukin-1 inhibit type I IFN expression and its downstream effects, demonstrating that a cross-regulatory network of cytokines operates during infectious diseases to provide protection with minimum damage to the host.
Figures

Similar articles
-
Confounding roles for type I interferons during bacterial and viral pathogenesis.Int Immunol. 2013 Dec;25(12):663-9. doi: 10.1093/intimm/dxt050. Epub 2013 Oct 24. Int Immunol. 2013. PMID: 24158954 Free PMC article. Review.
-
The Roles of Type I Interferon in Co-infections With Parasites and Viruses, Bacteria, or Other Parasites.Front Immunol. 2020 Oct 26;11:1805. doi: 10.3389/fimmu.2020.01805. eCollection 2020. Front Immunol. 2020. PMID: 33193291 Free PMC article. Review.
-
Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver's Seat.Front Immunol. 2019 Apr 12;10:778. doi: 10.3389/fimmu.2019.00778. eCollection 2019. Front Immunol. 2019. PMID: 31031767 Free PMC article. Review.
-
Type III IFNs: Beyond antiviral protection.Semin Immunol. 2019 Jun;43:101303. doi: 10.1016/j.smim.2019.101303. Semin Immunol. 2019. PMID: 31771761 Free PMC article. Review.
-
Host genetics: deciphering the variability in susceptibility to infections.Clin Microbiol Infect. 2014 Dec;20(12):1235-6. doi: 10.1111/1469-0691.12789. Epub 2014 Dec 12. Clin Microbiol Infect. 2014. PMID: 25274203 No abstract available.
Cited by
-
Short and Medium Chain Fatty Acids in a Cohort of Naïve Multiple Sclerosis Patients: Pre- and Post-Interferon Beta Treatment Assessment.Biologics. 2024 Nov 15;18:349-361. doi: 10.2147/BTT.S489523. eCollection 2024. Biologics. 2024. PMID: 39569059 Free PMC article.
-
Identification of novel markers for neuroblastoma immunoclustering using machine learning.Front Immunol. 2024 Nov 4;15:1446273. doi: 10.3389/fimmu.2024.1446273. eCollection 2024. Front Immunol. 2024. PMID: 39559348 Free PMC article.
-
Enhanced antiviral defense against begomoviral infection in Nicotiana benthamiana through strategic utilization of fluorescent carbon quantum dots to activate plant immunity.J Nanobiotechnology. 2024 Nov 14;22(1):707. doi: 10.1186/s12951-024-02994-4. J Nanobiotechnology. 2024. PMID: 39543670 Free PMC article.
-
Effect of chitosan nanogels loaded with vancomycin and gamma interferon on TNF-α gene expression in macrophage cell line activated with methicillin-resistant Staphylococcus aureus (MRSA).Iran J Microbiol. 2024 Oct;16(5):614-623. doi: 10.18502/ijm.v16i5.16794. Iran J Microbiol. 2024. PMID: 39534300 Free PMC article.
-
Endosomes serve as signaling platforms for RIG-I ubiquitination and activation.Sci Adv. 2024 Nov 8;10(45):eadq0660. doi: 10.1126/sciadv.adq0660. Epub 2024 Nov 6. Sci Adv. 2024. PMID: 39504361 Free PMC article.
References
-
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. - PubMed
-
- Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. - PubMed
-
- Witte K, Witte E, Sabat R, Wolk K. IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties. Cytokine Growth Factor Rev. 2010;21:237–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


