Motor contributions to the temporal precision of auditory attention
- PMID: 25314898
- PMCID: PMC4199392
- DOI: 10.1038/ncomms6255
Motor contributions to the temporal precision of auditory attention
Abstract
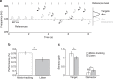
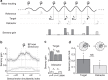
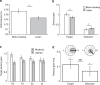
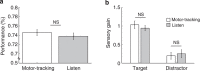
In temporal-or dynamic-attending theory, it is proposed that motor activity helps to synchronize temporal fluctuations of attention with the timing of events in a task-relevant stream, thus facilitating sensory selection. Here we develop a mechanistic behavioural account for this theory by asking human participants to track a slow reference beat, by noiseless finger pressing, while extracting auditory target tones delivered on-beat and interleaved with distractors. We find that overt rhythmic motor activity improves the segmentation of auditory information by enhancing sensitivity to target tones while actively suppressing distractor tones. This effect is triggered by cyclic fluctuations in sensory gain locked to individual motor acts, scales parametrically with the temporal predictability of sensory events and depends on the temporal alignment between motor and attention fluctuations. Together, these findings reveal how top-down influences associated with a rhythmic motor routine sharpen sensory representations, enacting auditory 'active sensing'.
Figures
Similar articles
-
Motor origin of temporal predictions in auditory attention.Proc Natl Acad Sci U S A. 2017 Oct 17;114(42):E8913-E8921. doi: 10.1073/pnas.1705373114. Epub 2017 Oct 2. Proc Natl Acad Sci U S A. 2017. PMID: 28973923 Free PMC article.
-
Push-pull competition between bottom-up and top-down auditory attention to natural soundscapes.Elife. 2020 Mar 20;9:e52984. doi: 10.7554/eLife.52984. Elife. 2020. PMID: 32196457 Free PMC article.
-
Neural tracking of the musical beat is enhanced by low-frequency sounds.Proc Natl Acad Sci U S A. 2018 Aug 7;115(32):8221-8226. doi: 10.1073/pnas.1801421115. Epub 2018 Jul 23. Proc Natl Acad Sci U S A. 2018. PMID: 30037989 Free PMC article.
-
Assessing the effect of sound complexity on the audiotactile cross-modal dynamic capture task.Q J Exp Psychol (Hove). 2010 Apr;63(4):694-704. doi: 10.1080/17470210903068989. Epub 2009 Aug 11. Q J Exp Psychol (Hove). 2010. PMID: 19672794
-
Neuronal oscillations as a mechanistic substrate of auditory temporal prediction.Ann N Y Acad Sci. 2015 Mar;1337(1):26-31. doi: 10.1111/nyas.12629. Ann N Y Acad Sci. 2015. PMID: 25773613 Free PMC article. Review.
Cited by
-
The Motor of Time: Coupling Action to Temporally Predictable Events Heightens Perception.Adv Exp Med Biol. 2024;1455:199-213. doi: 10.1007/978-3-031-60183-5_11. Adv Exp Med Biol. 2024. PMID: 38918353 Review.
-
A Second Introduction to the Neurobiology of Interval Timing.Adv Exp Med Biol. 2024;1455:3-23. doi: 10.1007/978-3-031-60183-5_1. Adv Exp Med Biol. 2024. PMID: 38918343 Review.
-
Evaluation of physiological response and synchronisation errors during synchronous and pseudosynchronous stimulation trials.Sci Rep. 2024 Apr 16;14(1):8814. doi: 10.1038/s41598-024-59477-7. Sci Rep. 2024. PMID: 38627479 Free PMC article.
-
Stationary stable cross-correlation pattern and task specific deviations in unresponsive wakefulness syndrome as well as clinically healthy subjects.PLoS One. 2024 Mar 15;19(3):e0300075. doi: 10.1371/journal.pone.0300075. eCollection 2024. PLoS One. 2024. PMID: 38489260 Free PMC article.
-
Neural dynamics of predictive timing and motor engagement in music listening.Sci Adv. 2024 Mar 8;10(10):eadi2525. doi: 10.1126/sciadv.adi2525. Epub 2024 Mar 6. Sci Adv. 2024. PMID: 38446888 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


