Methods for gene transfer to the central nervous system
- PMID: 25311922
- PMCID: PMC4519829
- DOI: 10.1016/B978-0-12-800149-3.00003-2
Methods for gene transfer to the central nervous system
Abstract
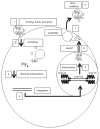
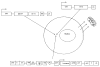
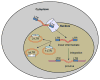
Gene transfer is an increasingly utilized approach for research and clinical applications involving the central nervous system (CNS). Vectors for gene transfer can be as simple as an unmodified plasmid, but more commonly involve complex modifications to viruses to make them suitable gene delivery vehicles. This chapter will explain how tools for CNS gene transfer have been derived from naturally occurring viruses. The current capabilities of plasmid, retroviral, adeno-associated virus, adenovirus, and herpes simplex virus vectors for CNS gene delivery will be described. These include both focal and global CNS gene transfer strategies, with short- or long-term gene expression. As is described in this chapter, an important aspect of any vector is the cis-acting regulatory elements incorporated into the vector genome that control when, where, and how the transgene is expressed.
Keywords: AAV; Adenovirus; CNS; Gene therapy; Lentivirus; Plasmid; Promoter; Vector.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



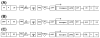

Similar articles
-
Introduction to Viral Vectors and Other Delivery Methods for Gene Therapy of the Nervous System.Methods Mol Biol. 2016;1382:3-18. doi: 10.1007/978-1-4939-3271-9_1. Methods Mol Biol. 2016. PMID: 26611575
-
Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review.
-
Adeno-associated virus and lentivirus vectors: a refined toolkit for the central nervous system.Curr Opin Virol. 2016 Dec;21:61-66. doi: 10.1016/j.coviro.2016.08.004. Epub 2016 Aug 23. Curr Opin Virol. 2016. PMID: 27559630 Review.
-
Gene transfer into the central nervous system in vivo using a recombinanat lentivirus vector.J Neurosci Res. 2002 Feb 1;67(3):363-71. doi: 10.1002/jnr.10137. J Neurosci Res. 2002. PMID: 11813241
-
Viral vectors for gene transfer: a review of their use in the treatment of human diseases.Drugs. 2000 Aug;60(2):249-71. doi: 10.2165/00003495-200060020-00002. Drugs. 2000. PMID: 10983732 Review.
Cited by
-
Trends on Novel Targets and Nanotechnology-Based Drug Delivery System in the Treatment of Parkinson's disease: Recent Advancement in Drug Development.Curr Drug Targets. 2024;25(15):987-1011. doi: 10.2174/0113894501312703240826070530. Curr Drug Targets. 2024. PMID: 39313872 Review.
-
The therapeutic implications of all-in-one AAV-delivered epigenome-editing platform in neurodegenerative disorders.Nat Commun. 2024 Aug 23;15(1):7259. doi: 10.1038/s41467-024-50515-6. Nat Commun. 2024. PMID: 39179542 Free PMC article.
-
Precision in Action: The Role of Clustered Regularly Interspaced Short Palindromic Repeats/Cas in Gene Therapies.Vaccines (Basel). 2024 Jun 7;12(6):636. doi: 10.3390/vaccines12060636. Vaccines (Basel). 2024. PMID: 38932365 Free PMC article. Review.
-
Emerging technology has a brilliant future: the CRISPR-Cas system for senescence, inflammation, and cartilage repair in osteoarthritis.Cell Mol Biol Lett. 2024 May 2;29(1):64. doi: 10.1186/s11658-024-00581-x. Cell Mol Biol Lett. 2024. PMID: 38698311 Free PMC article. Review.
-
Therapeutic bacteria and viruses to combat cancer: double-edged sword in cancer therapy: new insights for future.Cell Commun Signal. 2024 Apr 24;22(1):239. doi: 10.1186/s12964-024-01622-w. Cell Commun Signal. 2024. PMID: 38654309 Free PMC article. Review.
References
-
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, et al. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298(5594):846–850. - PubMed
-
- Adrover MF, Guyot-Revol V, Cheli VT, Blanco C, Vidal R, Alche L, et al. Hippocampal infection with HSV-1-derived vectors expressing an NMDAR1 antisense modifies behavior. Genes, Brain and Behavior. 2003;2(2):103–113. - PubMed
-
- Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Journal of Virology. 2006;80(19):9831–9836. http://dx.doi.org/10.1128/JVI.00878-06. - DOI - PMC - PubMed
-
- Akkina RK, Walton RM, Chen ML, Li QX, Planelles V, Chen IS. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. Journal of Virology. 1996;70(4):2581–2585. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials


