Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014-15 influenza season
- PMID: 25121712
- PMCID: PMC4584910
Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014-15 influenza season
Abstract
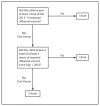
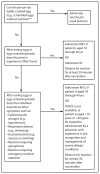
This report updates the 2013 recommendations by the Advisory Committee on Immunization Practices (ACIP) regarding use of seasonal influenza vaccines. Updated information for the 2014-15 influenza season includes 1) antigenic composition of U.S. seasonal influenza vaccines; 2) vaccine dose considerations for children aged 6 months through 8 years; and 3) a preference for the use, when immediately available, of live attenuated influenza vaccine (LAIV) for healthy children aged 2 through 8 years, to be implemented as feasible for the 2014-15 season but not later than the 2015-16 season. Information regarding issues related to influenza vaccination not addressed in this report is available in the 2013 ACIP seasonal influenza recommendations.
Figures
Similar articles
-
Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2022-23 Influenza Season.MMWR Recomm Rep. 2022 Aug 26;71(1):1-28. doi: 10.15585/mmwr.rr7101a1. MMWR Recomm Rep. 2022. PMID: 36006864 Free PMC article.
-
Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season.MMWR Morb Mortal Wkly Rep. 2015 Aug 7;64(30):818-25. doi: 10.15585/mmwr.mm6430a3. MMWR Morb Mortal Wkly Rep. 2015. PMID: 26247435 Free PMC article.
-
Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021-22 Influenza Season.MMWR Recomm Rep. 2021 Aug 27;70(5):1-28. doi: 10.15585/mmwr.rr7005a1. MMWR Recomm Rep. 2021. PMID: 34448800 Free PMC article.
-
Updates on Influenza Vaccination in Children.Infect Dis Clin North Am. 2018 Mar;32(1):75-89. doi: 10.1016/j.idc.2017.11.005. Infect Dis Clin North Am. 2018. PMID: 29406978 Review.
-
Revisiting live attenuated influenza vaccine efficacy among children in developing countries.Vaccine. 2023 Jan 27;41(5):1009-1017. doi: 10.1016/j.vaccine.2022.12.058. Epub 2023 Jan 4. Vaccine. 2023. PMID: 36604216 Review.
Cited by
-
Safety and immunogenicity of a quadrivalent, inactivated, split-virion influenza vaccine (IIV4-W) in healthy people aged 3-60 years: a phase III randomized clinical noninferiority trial.Hum Vaccin Immunother. 2022 Nov 30;18(5):2079924. doi: 10.1080/21645515.2022.2079924. Epub 2022 Jun 17. Hum Vaccin Immunother. 2022. PMID: 35714276 Free PMC article. Clinical Trial.
-
Identification and differential expression of microRNAs in Madin-Darby canine kidney cells with high and low tumorigenicities.Genes Genomics. 2022 Feb;44(2):187-196. doi: 10.1007/s13258-021-01177-x. Epub 2022 Jan 20. Genes Genomics. 2022. PMID: 35048333
-
Longitudinal Pathways to Influenza Vaccination Vary With Socio-Structural Disadvantages.Ann Behav Med. 2022 May 18;56(5):472-483. doi: 10.1093/abm/kaab087. Ann Behav Med. 2022. PMID: 34559192 Free PMC article.
-
City-wide school-located influenza vaccination: A retrospective cohort study.Vaccine. 2021 Oct 8;39(42):6302-6307. doi: 10.1016/j.vaccine.2021.08.099. Epub 2021 Sep 14. Vaccine. 2021. PMID: 34535312 Free PMC article.
-
Bivalent vaccination with NA1 and NA2 neuraminidase virus-like particles is protective against challenge with H1N1 and H3N2 influenza A viruses in a murine model.Virology. 2021 Oct;562:197-208. doi: 10.1016/j.virol.2021.08.001. Epub 2021 Aug 5. Virology. 2021. PMID: 34375782 Free PMC article.
References
-
- CDC. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013–2014. MMWR. 2013;62(RR-07) - PubMed
-
- Ahmed F. US Advisory Committee on Immunization Practices (ACIP) handbook for developing evidence-based recommendations. Version 1.2. Atlanta, GA: US Department of Health and Human Services, CDC; 2013.
-
- Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J. 2008;27:744–8. - PubMed
-
- Ochiai H, Shibata M, Kamimura K, Niwayama S. Evaluation of the efficacy of split-product trivalent A(H1N1), A(H3N2), and B influenza vaccines: reactogenicity, immunogenicity and persistence of antibodies following two doses of vaccines. Microbiol Immunol. 1986;30:1141–9. - PubMed
-
- Kunzel W, Glathe H, Engelmann H, Van Hoecke C. Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine. 1996;14:1108–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


