rBCG30-induced immunity and cross-protection against Mycobacterium leprae challenge are enhanced by boosting with the Mycobacterium tuberculosis 30-kilodalton antigen 85B
- PMID: 25001602
- PMCID: PMC4187824
- DOI: 10.1128/IAI.01499-13
rBCG30-induced immunity and cross-protection against Mycobacterium leprae challenge are enhanced by boosting with the Mycobacterium tuberculosis 30-kilodalton antigen 85B
Abstract
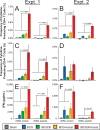
Leprosy remains a major global health problem and typically occurs in regions in which tuberculosis is endemic. Vaccines are needed that protect against both infections and do so better than the suboptimal Mycobacterium bovis BCG vaccine. Here, we evaluated rBCG30, a vaccine previously demonstrated to induce protection superior to that of BCG against Mycobacterium tuberculosis and Mycobacterium bovis challenge in animal models, for efficacy against Mycobacterium leprae challenge in a murine model of leprosy. rBCG30 overexpresses the M. tuberculosis 30-kDa major secretory protein antigen 85B, which is 85% homologous with the M. leprae homolog (r30ML). Mice were sham immunized or immunized intradermally with BCG or rBCG30 and challenged 2.5 months later by injection of viable M. leprae into each hind footpad. After 7 months, vaccine efficacy was assessed by enumerating the M. leprae bacteria per footpad. Both BCG and rBCG30 induced significant protection against M. leprae challenge. In the one experiment in which a comparison between BCG and rBCG30 was feasible, rBCG30 induced significantly greater protection than did BCG. Immunization of mice with purified M. tuberculosis or M. leprae antigen 85B also induced protection against M. leprae challenge but less so than BCG or rBCG30. Notably, boosting rBCG30 with M. tuberculosis antigen 85B significantly enhanced r30ML-specific immune responses, substantially more so than boosting BCG, and significantly augmented protection against M. leprae challenge. Thus, rBCG30, a vaccine that induces improved protection against M. tuberculosis, induces cross-protection against M. leprae that is comparable or potentially superior to that induced by BCG, and boosting rBCG30 with antigen 85B further enhances immune responses and protective efficacy.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Vaccines for Leprosy and Tuberculosis: Opportunities for Shared Research, Development, and Application.Front Immunol. 2018 Feb 26;9:308. doi: 10.3389/fimmu.2018.00308. eCollection 2018. Front Immunol. 2018. PMID: 29535713 Free PMC article. Review.
-
Extraordinarily few organisms of a live recombinant BCG vaccine against tuberculosis induce maximal cell-mediated and protective immunity.Vaccine. 2006 Jan 23;24(4):443-51. doi: 10.1016/j.vaccine.2005.08.001. Epub 2005 Aug 11. Vaccine. 2006. PMID: 16125825
-
Highly persistent and effective prime/boost regimens against tuberculosis that use a multivalent modified vaccine virus Ankara-based tuberculosis vaccine with interleukin-15 as a molecular adjuvant.Clin Vaccine Immunol. 2010 May;17(5):793-801. doi: 10.1128/CVI.00006-10. Epub 2010 Mar 31. Clin Vaccine Immunol. 2010. PMID: 20357059 Free PMC article.
-
A live attenuated BCG vaccine overexpressing multistage antigens Ag85B and HspX provides superior protection against Mycobacterium tuberculosis infection.Appl Microbiol Biotechnol. 2015 Dec;99(24):10587-95. doi: 10.1007/s00253-015-6962-x. Epub 2015 Sep 12. Appl Microbiol Biotechnol. 2015. PMID: 26363555
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Identification of Immunodominant Proteins of the Leishmania (Viannia) naiffi SubProteome as Pan-Specific Vaccine Targets against Leishmaniasis.Vaccines (Basel). 2023 Jun 21;11(7):1129. doi: 10.3390/vaccines11071129. Vaccines (Basel). 2023. PMID: 37514945 Free PMC article.
-
Recent Developments in Mycobacteria-Based Live Attenuated Vaccine Candidates for Tuberculosis.Biomedicines. 2022 Oct 29;10(11):2749. doi: 10.3390/biomedicines10112749. Biomedicines. 2022. PMID: 36359269 Free PMC article. Review.
-
A century of BCG vaccination: Immune mechanisms, animal models, non-traditional routes and implications for COVID-19.Front Immunol. 2022 Aug 26;13:959656. doi: 10.3389/fimmu.2022.959656. eCollection 2022. Front Immunol. 2022. PMID: 36091032 Free PMC article. Review.
-
Immunotherapy With 5, 15-DPP Mediates Macrophage M1 Polarization and Modulates Subsequent Mycobacterium tuberculosis Infectivity in rBCG30 Immunized Mice.Front Immunol. 2021 Oct 29;12:706727. doi: 10.3389/fimmu.2021.706727. eCollection 2021. Front Immunol. 2021. PMID: 34777338 Free PMC article.
-
Early innate and adaptive immune perturbations determine long-term severity of chronic virus and Mycobacterium tuberculosis coinfection.Immunity. 2021 Mar 9;54(3):526-541.e7. doi: 10.1016/j.immuni.2021.01.003. Epub 2021 Jan 29. Immunity. 2021. PMID: 33515487 Free PMC article.
References
-
- World Health Organization. 2013. Global leprosy: update on the 2012 situation. Wkly. Epidemiol. Rec. 88:365–379 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


