Intraoperative dorsal language network mapping by using single-pulse electrical stimulation
- PMID: 24615889
- PMCID: PMC6869787
- DOI: 10.1002/hbm.22479
Intraoperative dorsal language network mapping by using single-pulse electrical stimulation
Abstract
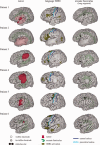
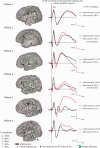
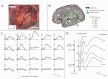
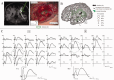
The preservation of language function during brain surgery still poses a challenge. No intraoperative methods have been established to monitor the language network reliably. We aimed to establish intraoperative language network monitoring by means of cortico-cortical evoked potentials (CCEPs). Subjects were six patients with tumors located close to the arcuate fasciculus (AF) in the language-dominant left hemisphere. Under general anesthesia, the anterior perisylvian language area (AL) was first defined by the CCEP connectivity patterns between the ventrolateral frontal and temporoparietal area, and also by presurgical neuroimaging findings. We then monitored the integrity of the language network by stimulating AL and by recording CCEPs from the posterior perisylvian language area (PL) consecutively during both general anesthesia and awake condition. High-frequency electrical stimulation (ES) performed during awake craniotomy confirmed language function at AL in all six patients. Despite an amplitude decline (≤32%) in two patients, CCEP monitoring successfully prevented persistent language impairment. After tumor removal, single-pulse ES was applied to the white matter tract beneath the floor of the removal cavity in five patients, in order to trace its connections into the language cortices. In three patients in whom high-frequency ES of the white matter produced naming impairment, this "eloquent" subcortical site directly connected AL and PL, judging from the latencies and distributions of cortico- and subcortico-cortical evoked potentials. In conclusion, this study provided the direct evidence that AL, PL, and AF constitute the dorsal language network. Intraoperative CCEP monitoring is clinically useful for evaluating the integrity of the language network.
Keywords: arcuate fasciculus; awake craniotomy; cortico-cortical evoked potential; dorsal language network; subcortico-cortical evoked potential.
Copyright © 2014 Wiley Periodicals, Inc.
Figures

Similar articles
-
Clinical impact of intraoperative CCEP monitoring in evaluating the dorsal language white matter pathway.Hum Brain Mapp. 2017 Apr;38(4):1977-1991. doi: 10.1002/hbm.23498. Epub 2017 Jan 23. Hum Brain Mapp. 2017. PMID: 28112455 Free PMC article.
-
Effects of propofol on cortico-cortical evoked potentials in the dorsal language white matter pathway.Clin Neurophysiol. 2021 Aug;132(8):1919-1926. doi: 10.1016/j.clinph.2021.04.021. Epub 2021 Jun 1. Clin Neurophysiol. 2021. PMID: 34182277
-
Intraoperative Corticocortical Evoked Potentials for Language Monitoring in Epilepsy Surgery.World Neurosurg. 2021 Jul;151:e109-e121. doi: 10.1016/j.wneu.2021.03.141. Epub 2021 Apr 2. World Neurosurg. 2021. PMID: 33819704
-
Intraoperative Brain Mapping by Cortico-Cortical Evoked Potential.Front Hum Neurosci. 2021 Feb 18;15:635453. doi: 10.3389/fnhum.2021.635453. eCollection 2021. Front Hum Neurosci. 2021. PMID: 33679353 Free PMC article. Review.
-
Intraoperative Direct Stimulation Identification and Preservation of Critical White Matter Tracts During Brain Surgery.World Neurosurg. 2021 Feb;146:64-74. doi: 10.1016/j.wneu.2020.10.100. Epub 2020 Oct 24. World Neurosurg. 2021. PMID: 33229311 Review.
Cited by
-
Effective connectivity relates seizure outcome to electrode placement in responsive neurostimulation.Brain Commun. 2024 Feb 22;6(1):fcae035. doi: 10.1093/braincomms/fcae035. eCollection 2024. Brain Commun. 2024. PMID: 38390255 Free PMC article.
-
Thalamic feedback shapes brain responses evoked by cortical stimulation in mice and humans.bioRxiv [Preprint]. 2024 Apr 12:2024.01.31.578243. doi: 10.1101/2024.01.31.578243. bioRxiv. 2024. PMID: 38352535 Free PMC article. Preprint.
-
ER-detect: a pipeline for robust detection of early evoked responses in BIDS-iEEG electrical stimulation data.bioRxiv [Preprint]. 2024 Jan 11:2024.01.09.574915. doi: 10.1101/2024.01.09.574915. bioRxiv. 2024. PMID: 38260687 Free PMC article. Preprint.
-
Flexible, scalable, high channel count stereo-electrode for recording in the human brain.Nat Commun. 2024 Jan 17;15(1):218. doi: 10.1038/s41467-023-43727-9. Nat Commun. 2024. PMID: 38233418 Free PMC article.
-
Guidelines for Awake Surgery.Neurol Med Chir (Tokyo). 2024;64(1):1-27. doi: 10.2176/jns-nmc.2023-0111. Neurol Med Chir (Tokyo). 2024. PMID: 38220155 Free PMC article. No abstract available.
References
-
- Berger MS, Kincaid J, Ojemann GA, Lettich E (1989): Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors. Neurosurgery 25:786–792. - PubMed
-
- Bizzi A, Nava S, Ferré F, Castelli G, Aquino D, Ciaraffa F, Broggi G, DiMeco F, Piacentini S (2012): Aphasia induced by gliomas growing in the ventrolateral frontal region: Assessment with diffusion MR tractography, functional MR imaging and neuropsychology. Cortex 48:255–272. - PubMed
-
- Catani M, Dell'acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A, Murphy DG, Thiebaut de Schotten M (2012): Beyond cortical localization in clinico‐anatomical correlation. Cortex 48:1262–1287. - PubMed
-
- Catenoix H, Magnin M, Guénot M, Isnard J, Mauguiére F, Ryvlin P (2005): Hippocampal‐orbitofrontal connectivity in human: An electrical stimulation study. Clin Neurophysiol 116:1779–1784. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources


