"Cold" X5 Hairlaser™ used to treat male androgenic alopecia and hair growth: an uncontrolled pilot study
- PMID: 24559020
- PMCID: PMC3974052
- DOI: 10.1186/1756-0500-7-103
"Cold" X5 Hairlaser™ used to treat male androgenic alopecia and hair growth: an uncontrolled pilot study
Abstract
Background: Various trials have been conducted on the management and treatment of androgenic alopecia (AGA) or male pattern hair loss using a variety of laser and light sources.
Methods: For this feasibility study, the population was composed of males between the ages of 20 and 60 years who have been experiencing active hair loss within the last 12 months and the diagnosis of AGA. They also had a Norwood-Hamilton classification of 3, 3A, 3 V, 4, 4A, or 5 for the hair thinning patterns and skin type I, II, III, or IV on the Fitzpatrick skin type scale. This two-arm randomized, parallel group study design employed stratifying randomization to balance treatment assignment within three investigational centers with at least 2 subjects enrolled in each Fitzpatrick skin type.
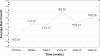
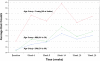
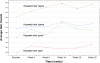
Results: A statistically significant positive trend in hair growth was observed from this pilot study, to evaluate the efficacy of the novel cold X5 hairlaser device for treating male androgenic alopecia. From the repeated measures analysis of variance, difference in mean hair counts over time was statistically significant (F = 7.70; p-value < 0.0001). Subsequent, linear regression of mean hair counts at each time point was performed, and post-hoc analysis found an increasing trend of hair growth over time that was statistically significant (p-value < 0.0001) with the estimated slope of 1.406. Increased hair counts from the baseline to the end of the 26-week period were found to be strongly significant (p-value = 0.0003).
Conclusion: Albeit, sham device failure and resultant missing data from the control group, the positive trend hair growth, was observed due to the chronic use of X5hairlaser device. This positive benefit while in full agreement with other low laser hair devices requires intensive further investigation.
Trial registration: NCT02067260.
Figures


Similar articles
-
Low-level laser therapy for the treatment of androgenic alopecia: a review.Lasers Med Sci. 2018 Feb;33(2):425-434. doi: 10.1007/s10103-017-2385-5. Epub 2017 Dec 21. Lasers Med Sci. 2018. PMID: 29270707 Review.
-
HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial.Clin Drug Investig. 2009;29(5):283-92. doi: 10.2165/00044011-200929050-00001. Clin Drug Investig. 2009. PMID: 19366270 Clinical Trial.
-
Promising therapies for treating and/or preventing androgenic alopecia.Skin Therapy Lett. 2012 Jun;17(6):1-4. Skin Therapy Lett. 2012. PMID: 22735503 Review.
-
Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: a 24-week, randomized, double-blind, sham device-controlled trial.Lasers Med Sci. 2019 Aug;34(6):1107-1114. doi: 10.1007/s10103-018-02699-9. Epub 2018 Dec 19. Lasers Med Sci. 2019. PMID: 30569416 Clinical Trial.
-
The growth of human scalp hair mediated by visible red light laser and LED sources in males.Lasers Surg Med. 2013 Oct;45(8):487-95. doi: 10.1002/lsm.22173. Lasers Surg Med. 2013. PMID: 24078483 Clinical Trial.
Cited by
-
Efficacy and Safety of Laser Therapy and Phototherapy in Cicatricial and NonCicatricial Alopecia: A Systematic Review Study.Health Sci Rep. 2024 Nov 4;7(11):e70180. doi: 10.1002/hsr2.70180. eCollection 2024 Nov. Health Sci Rep. 2024. PMID: 39502132 Free PMC article. Review.
-
Effect of Skin Type on Efficacy of Laser Treatment for Androgenetic Alopecia: A Review of the Literature.Skin Appendage Disord. 2023 Oct;9(5):317-324. doi: 10.1159/000528518. Epub 2023 Jul 3. Skin Appendage Disord. 2023. PMID: 37900781 Free PMC article. Review.
-
Comparing Current Therapeutic Modalities of Androgenic Alopecia: A Literature Review of Clinical Trials.Cureus. 2023 Jul 31;15(7):e42768. doi: 10.7759/cureus.42768. eCollection 2023 Jul. Cureus. 2023. PMID: 37663989 Free PMC article. Review.
-
A Systematic Review and Meta-analysis of Randomized Controlled Trials of United States Food and Drug Administration-Approved, Home-use, Low-Level Light/Laser Therapy Devices for Pattern Hair Loss: Device Design and Technology.J Clin Aesthet Dermatol. 2021 Nov;14(11):E64-E75. J Clin Aesthet Dermatol. 2021. PMID: 34980962 Free PMC article.
-
Low-level laser therapy for the treatment of androgenic alopecia: a review.Lasers Med Sci. 2018 Feb;33(2):425-434. doi: 10.1007/s10103-017-2385-5. Epub 2017 Dec 21. Lasers Med Sci. 2018. PMID: 29270707 Review.
References
-
- Liang B, Yang C, Zuo X, Li Y, Ding Y, Sheng Y, Zhou F, Cheng H, Zheng X, Chen G, Zhu Z, Zhu J, Fu X, Wang T, Dong Y, Duan D, Tang X, Tang H, Gao J, Sun L, Yang S, Zhang X. Genetic variants at 20p11 confer risk to androgenetic alopeciain the Chinese Han population. PLoS One. 2013;8(8):e71771. doi: 10.1371/journal.pone.0071771. - DOI - PMC - PubMed
-
- Chin EY. Androgenetic alopecia (male pattern hair loss) in the United States: what treatments should primary care providers recommend? J Am Assoc Nurs Pract. 2013;25(8):395–401. - PubMed
-
- Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, Price VH, Van Neste D, Roberts JL, Hordinsky M, Shapiro J, Binkowitz B, Gormley GJ. Finasteride Male Pattern Hair Loss Study Group. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39(4 Pt 1):578–589. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


