Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor
- PMID: 24172901
- PMCID: PMC5389864
- DOI: 10.1038/nature12711
Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor
Abstract
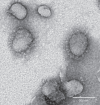
The 2002-3 pandemic caused by severe acute respiratory syndrome coronavirus (SARS-CoV) was one of the most significant public health events in recent history. An ongoing outbreak of Middle East respiratory syndrome coronavirus suggests that this group of viruses remains a key threat and that their distribution is wider than previously recognized. Although bats have been suggested to be the natural reservoirs of both viruses, attempts to isolate the progenitor virus of SARS-CoV from bats have been unsuccessful. Diverse SARS-like coronaviruses (SL-CoVs) have now been reported from bats in China, Europe and Africa, but none is considered a direct progenitor of SARS-CoV because of their phylogenetic disparity from this virus and the inability of their spike proteins to use the SARS-CoV cellular receptor molecule, the human angiotensin converting enzyme II (ACE2). Here we report whole-genome sequences of two novel bat coronaviruses from Chinese horseshoe bats (family: Rhinolophidae) in Yunnan, China: RsSHC014 and Rs3367. These viruses are far more closely related to SARS-CoV than any previously identified bat coronaviruses, particularly in the receptor binding domain of the spike protein. Most importantly, we report the first recorded isolation of a live SL-CoV (bat SL-CoV-WIV1) from bat faecal samples in Vero E6 cells, which has typical coronavirus morphology, 99.9% sequence identity to Rs3367 and uses ACE2 from humans, civets and Chinese horseshoe bats for cell entry. Preliminary in vitro testing indicates that WIV1 also has a broad species tropism. Our results provide the strongest evidence to date that Chinese horseshoe bats are natural reservoirs of SARS-CoV, and that intermediate hosts may not be necessary for direct human infection by some bat SL-CoVs. They also highlight the importance of pathogen-discovery programs targeting high-risk wildlife groups in emerging disease hotspots as a strategy for pandemic preparedness.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
Bats as animal reservoirs for the SARS coronavirus: hypothesis proved after 10 years of virus hunting.Virol Sin. 2013 Dec;28(6):315-7. doi: 10.1007/s12250-013-3402-x. Epub 2013 Oct 30. Virol Sin. 2013. PMID: 24174406 Free PMC article.
Similar articles
-
Evolutionary Arms Race between Virus and Host Drives Genetic Diversity in Bat Severe Acute Respiratory Syndrome-Related Coronavirus Spike Genes.J Virol. 2020 Sep 29;94(20):e00902-20. doi: 10.1128/JVI.00902-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32699095 Free PMC article.
-
Difference in receptor usage between severe acute respiratory syndrome (SARS) coronavirus and SARS-like coronavirus of bat origin.J Virol. 2008 Feb;82(4):1899-907. doi: 10.1128/JVI.01085-07. Epub 2007 Dec 12. J Virol. 2008. PMID: 18077725 Free PMC article.
-
Severe Acute Respiratory Syndrome (SARS) Coronavirus ORF8 Protein Is Acquired from SARS-Related Coronavirus from Greater Horseshoe Bats through Recombination.J Virol. 2015 Oct;89(20):10532-47. doi: 10.1128/JVI.01048-15. Epub 2015 Aug 12. J Virol. 2015. PMID: 26269185 Free PMC article.
-
A review of studies on animal reservoirs of the SARS coronavirus.Virus Res. 2008 Apr;133(1):74-87. doi: 10.1016/j.virusres.2007.03.012. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451830 Free PMC article. Review.
-
Molecular epidemiology, evolution and phylogeny of SARS coronavirus.Infect Genet Evol. 2019 Jul;71:21-30. doi: 10.1016/j.meegid.2019.03.001. Epub 2019 Mar 4. Infect Genet Evol. 2019. PMID: 30844511 Free PMC article. Review.
Cited by
-
Design of customized coronavirus receptors.Nature. 2024 Oct 30. doi: 10.1038/s41586-024-08121-5. Online ahead of print. Nature. 2024. PMID: 39478224
-
The first report on detecting SARS-CoV-2 inside bacteria of the human gut microbiome: A case series on asymptomatic family members and a child with COVID-19.F1000Res. 2024 Oct 16;11:135. doi: 10.12688/f1000research.77421.2. eCollection 2022. F1000Res. 2024. PMID: 39464247 Free PMC article.
-
An Analysis of Combined Molecular Weight and Hydrophobicity Similarity between the Amino Acid Sequences of Spike Protein Receptor Binding Domains of Betacoronaviruses and Functionally Similar Sequences from Other Virus Families.Microorganisms. 2024 Oct 5;12(10):2021. doi: 10.3390/microorganisms12102021. Microorganisms. 2024. PMID: 39458330 Free PMC article.
-
Discovery of a novel benzimidazole conjugated quinazolinone derivative as a promising SARS-CoV-2 3CL protease inhibitor.RSC Adv. 2024 Oct 24;14(46):33820-33829. doi: 10.1039/d4ra03267e. eCollection 2024 Oct 23. RSC Adv. 2024. PMID: 39450066 Free PMC article.
-
Broad cross neutralizing antibodies against sarbecoviruses generated by SARS-CoV-2 infection and vaccination in humans.NPJ Vaccines. 2024 Oct 22;9(1):195. doi: 10.1038/s41541-024-00997-8. NPJ Vaccines. 2024. PMID: 39438493 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


