Neural plasticity and proliferation in the generation of antidepressant effects: hippocampal implication
- PMID: 23862076
- PMCID: PMC3703717
- DOI: 10.1155/2013/537265
Neural plasticity and proliferation in the generation of antidepressant effects: hippocampal implication
Abstract
It is widely accepted that changes underlying depression and antidepressant-like effects involve not only alterations in the levels of neurotransmitters as monoamines and their receptors in the brain, but also structural and functional changes far beyond. During the last two decades, emerging theories are providing new explanations about the neurobiology of depression and the mechanism of action of antidepressant strategies based on cellular changes at the CNS level. The neurotrophic/plasticity hypothesis of depression, proposed more than a decade ago, is now supported by multiple basic and clinical studies focused on the role of intracellular-signalling cascades that govern neural proliferation and plasticity. Herein, we review the state-of-the-art of the changes in these signalling pathways which appear to underlie both depressive disorders and antidepressant actions. We will especially focus on the hippocampal cellularity and plasticity modulation by serotonin, trophic factors as brain-derived neurotrophic factor (BDNF), and vascular endothelial growth factor (VEGF) through intracellular signalling pathways-cAMP, Wnt/ β -catenin, and mTOR. Connecting the classic monoaminergic hypothesis with proliferation/neuroplasticity-related evidence is an appealing and comprehensive attempt for improving our knowledge about the neurobiological events leading to depression and associated to antidepressant therapies.
Figures
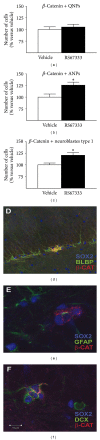
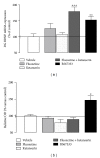
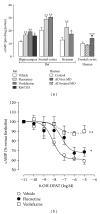
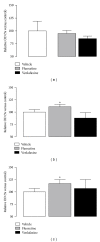
Similar articles
-
The hippocampus, neurotrophic factors and depression: possible implications for the pharmacotherapy of depression.CNS Drugs. 2011 Nov 1;25(11):913-31. doi: 10.2165/11595900-000000000-00000. CNS Drugs. 2011. PMID: 22054117 Review.
-
Signaling pathways involved in antidepressant-induced cell proliferation and synaptic plasticity.Curr Pharm Des. 2014;20(23):3776-94. doi: 10.2174/13816128113196660736. Curr Pharm Des. 2014. PMID: 24180397 Review.
-
Antidepressant-like effects of 3,6'-disinapoyl sucrose on hippocampal neuronal plasticity and neurotrophic signal pathway in chronically mild stressed rats.Neurochem Int. 2010 Feb;56(3):461-5. doi: 10.1016/j.neuint.2009.12.004. Epub 2009 Dec 14. Neurochem Int. 2010. PMID: 20018220
-
Changes in Hippocampal Plasticity in Depression and Therapeutic Approaches Influencing These Changes.Neural Plast. 2020 Nov 26;2020:8861903. doi: 10.1155/2020/8861903. eCollection 2020. Neural Plast. 2020. PMID: 33293948 Free PMC article. Review.
-
New strategies in the development of antidepressants: towards the modulation of neuroplasticity pathways.Curr Pharm Des. 2011;17(5):521-33. doi: 10.2174/138161211795164086. Curr Pharm Des. 2011. PMID: 21375480 Review.
Cited by
-
Visual EMDR stimulation mitigates acute varied stress effects on morphology of hippocampal neurons in male Wistar rats.Front Psychiatry. 2024 May 13;15:1396550. doi: 10.3389/fpsyt.2024.1396550. eCollection 2024. Front Psychiatry. 2024. PMID: 38803673 Free PMC article.
-
The Impact of BDNF, NTRK2, NGFR, CREB1, GSK3B, AKT, MAPK1, MTOR, PTEN, ARC, and SYN1 Genetic Polymorphisms in Antidepressant Treatment Response Phenotypes.Int J Mol Sci. 2023 Apr 4;24(7):6758. doi: 10.3390/ijms24076758. Int J Mol Sci. 2023. PMID: 37047730 Free PMC article.
-
Antidepressants for the treatment of depression in people with cancer.Cochrane Database Syst Rev. 2023 Mar 31;3(3):CD011006. doi: 10.1002/14651858.CD011006.pub4. Cochrane Database Syst Rev. 2023. PMID: 36999619 Free PMC article. Review.
-
Neurotoxins Acting at Synaptic Sites: A Brief Review on Mechanisms and Clinical Applications.Toxins (Basel). 2022 Dec 27;15(1):18. doi: 10.3390/toxins15010018. Toxins (Basel). 2022. PMID: 36668838 Free PMC article. Review.
-
Honokiol improves depression-like behaviors in rats by HIF-1α- VEGF signaling pathway activation.Front Pharmacol. 2022 Aug 25;13:968124. doi: 10.3389/fphar.2022.968124. eCollection 2022. Front Pharmacol. 2022. PMID: 36091747 Free PMC article.
References
-
- WHO. The world health report. 2001.
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) APA Press; 1994.
-
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28(4):676–691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous


