Selective serotonin reuptake inhibitors for premenstrual syndrome
- PMID: 23744611
- PMCID: PMC7073417
- DOI: 10.1002/14651858.CD001396.pub3
Selective serotonin reuptake inhibitors for premenstrual syndrome
Update in
-
Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder.Cochrane Database Syst Rev. 2024 Aug 14;8(8):CD001396. doi: 10.1002/14651858.CD001396.pub4. Cochrane Database Syst Rev. 2024. PMID: 39140320 Review.
Abstract
Background: Premenstrual syndrome (PMS) is a common cause of physical, psychological and social problems in women of reproductive age. The key characteristic of PMS is the timing of symptoms, which occur only during the two weeks leading up to menstruation (the luteal phase of the menstrual cycle). Selective serotonin reuptake inhibitors (SSRIs) are increasingly used as first line therapy for PMS. SSRIs can be taken either in the luteal phase or else continuously (every day). SSRIs are generally considered to be effective for reducing premenstrual symptoms but they can cause adverse effects.
Objectives: The objective of this review was to evaluate the effectiveness and safety of SSRIs for treating premenstrual syndrome.
Search methods: Electronic searches for relevant randomised controlled trials (RCTs) were undertaken in the Cochrane Menstrual Disorders and Subfertility Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE, EMBASE, PsycINFO, and CINAHL (February 2013). Where insufficient data were presented in a report, attempts were made to contact the original authors for further details.
Selection criteria: Studies were considered in which women with a prospective diagnosis of PMS, PMDD or late luteal phase dysphoric disorder (LPDD) were randomised to receive SSRIs or placebo for the treatment of premenstrual syndrome.
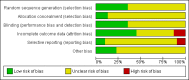
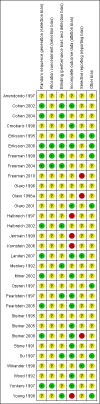
Data collection and analysis: Two review authors independently selected the studies, assessed eligible studies for risk of bias, and extracted data on premenstrual symptoms and adverse effects. Studies were pooled using random-effects models. Standardised mean differences (SMDs) with 95% confidence intervals (CIs) were calculated for premenstrual symptom scores, using separate analyses for different types of continuous data (that is end scores and change scores). Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for dichotomous outcomes. Analyses were stratified by type of drug administration (luteal or continuous) and by drug dose (low, medium, or high). We calculated the number of women who would need to be taking a moderate dose of SSRI in order to cause one additional adverse event (number needed to harm: NNH). The overall quality of the evidence for the main findings was assessed using the GRADE working group methods.
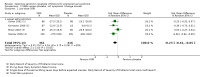
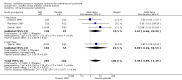
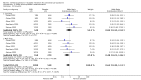
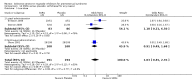
Main results: Thirty-one RCTs were included in the review. They compared fluoxetine, paroxetine, sertraline, escitalopram and citalopram versus placebo. SSRIs reduced overall self-rated symptoms significantly more effectively than placebo. The effect size was moderate when studies reporting end scores were pooled (for moderate dose SSRIs: SMD -0.65, 95% CI -0.46 to -0.84, nine studies, 1276 women; moderate heterogeneity (I(2) = 58%), low quality evidence). The effect size was small when studies reporting change scores were pooled (for moderate dose SSRIs: SMD -0.36, 95% CI -0.20 to -0.51, four studies, 657 women; low heterogeneity (I(2)=29%), moderate quality evidence).SSRIs were effective for symptom relief whether taken only in the luteal phase or continuously, with no clear evidence of a difference in effectiveness between these modes of administration. However, few studies directly compared luteal and continuous regimens and more evidence is needed on this question.Withdrawals due to adverse effects were significantly more likely to occur in the SSRI group (moderate dose: OR 2.55, 95% CI 1.84 to 3.53, 15 studies, 2447 women; no heterogeneity (I(2) = 0%), moderate quality evidence). The most common side effects associated with a moderate dose of SSRIs were nausea (NNH = 7), asthenia or decreased energy (NNH = 9), somnolence (NNH = 13), fatigue (NNH = 14), decreased libido (NNH = 14) and sweating (NNH = 14). In secondary analyses, SSRIs were effective for treating specific types of symptoms (that is psychological, physical and functional symptoms, and irritability). Adverse effects were dose-related.The overall quality of the evidence was low to moderate, the main weakness in the included studies being poor reporting of methods. Heterogeneity was low or absent for most outcomes, though (as noted above) there was moderate heterogeneity for one of the primary analyses.
Authors' conclusions: SSRIs are effective in reducing the symptoms of PMS, whether taken in the luteal phase only or continuously. Adverse effects are relatively frequent, the most common being nausea and asthenia. Adverse effects are dose-dependent.
Conflict of interest statement
Julie Brown and Jane Marjoribanks have no conflict of interest.
PMS O'Brien has been funded over many years for research personnel, consultancies, lectures and conferences by the following companies: Bayer Schering, Hoechst Marion Roussel Ltd, Shire Pharmaceuticals, Smith Kline Beecham, Eli Lilly, Searle, Sanofi Winthrop, Zeneca, Organon, Solvay Pharmaceuticals and Novo Nordisk.
KM Wyatt has no conflict of interest.
Figures























































Update of
-
Selective serotonin reuptake inhibitors for premenstrual syndrome.Cochrane Database Syst Rev. 2009 Apr 15;(2):CD001396. doi: 10.1002/14651858.CD001396.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2013 Jun 07;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3. PMID: 19370564 Updated. Review.
Similar articles
-
Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder.Cochrane Database Syst Rev. 2024 Aug 14;8(8):CD001396. doi: 10.1002/14651858.CD001396.pub4. Cochrane Database Syst Rev. 2024. PMID: 39140320 Review.
-
Selective serotonin reuptake inhibitors for premenstrual syndrome.Cochrane Database Syst Rev. 2009 Apr 15;(2):CD001396. doi: 10.1002/14651858.CD001396.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2013 Jun 07;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3. PMID: 19370564 Updated. Review.
-
Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome.Cochrane Database Syst Rev. 2017 Mar 3;3(3):CD010503. doi: 10.1002/14651858.CD010503.pub2. Cochrane Database Syst Rev. 2017. PMID: 28257559 Free PMC article. Review.
-
Acupuncture and acupressure for premenstrual syndrome.Cochrane Database Syst Rev. 2018 Aug 14;8(8):CD005290. doi: 10.1002/14651858.CD005290.pub2. Cochrane Database Syst Rev. 2018. PMID: 30105749 Free PMC article.
-
Selective serotonin reuptake inhibitors for premenstrual syndrome.Cochrane Database Syst Rev. 2002;(4):CD001396. doi: 10.1002/14651858.CD001396. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2009 Apr 15;(2):CD001396. doi: 10.1002/14651858.CD001396.pub2. PMID: 12519554 Updated. Review.
Cited by
-
Prevalence of Moderate-to-Severe Premenstrual Syndrome or Premenstrual Dysphoric Disorder and Associated Variables Among Young Women in a Tertiary Health Care Center in Eastern India.Cureus. 2024 Sep 14;16(9):e69400. doi: 10.7759/cureus.69400. eCollection 2024 Sep. Cureus. 2024. PMID: 39403645 Free PMC article.
-
Cumulative stressor exposure predicts menstrual cycle affective changes in a transdiagnostic outpatient sample with past-month suicidal ideation.Psychol Med. 2024 Oct 14;54(13):1-12. doi: 10.1017/S0033291724001661. Online ahead of print. Psychol Med. 2024. PMID: 39397675 Free PMC article.
-
Endocrinological Treatment Targets for Depressive Disorder.Adv Exp Med Biol. 2024;1456:3-25. doi: 10.1007/978-981-97-4402-2_1. Adv Exp Med Biol. 2024. PMID: 39261421 Review.
-
Practical diagnosis and treatment of premenstrual syndrome and premenstrual dysphoric disorder by psychiatrists and obstetricians/gynecologists in Japan.PCN Rep. 2024 Aug 15;3(3):e234. doi: 10.1002/pcn5.234. eCollection 2024 Sep. PCN Rep. 2024. PMID: 39149567 Free PMC article.
-
Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder.Cochrane Database Syst Rev. 2024 Aug 14;8(8):CD001396. doi: 10.1002/14651858.CD001396.pub4. Cochrane Database Syst Rev. 2024. PMID: 39140320 Review.
References
References to studies included in this review
Arrendondo 1997 {published data only}
-
- Arredondo‐Soberon F, Freeman EW, Sondheimer SJ. Relationship and response of food cravings and depression to sertraline in patients with premenstrual syndrome. Fertility and Sterility 1997;October:S28.
Cohen 2002 {published data only}
-
- Cohen L, Miner C, Brown E, Dillon J. Efficacy of intermittent fluoxetine dosing in premenstrual dysphoric disorder (PMDD). European Neuropsychopharmacology 2001;11 Suppl 3:210.
-
- Cohen L, Miner C, Brown E, Freeman E, Halbreich U, Sundell K, McCray S. Premenstrual daily fluoxetine for premenstrual dysphoric disorder: A placebo controlled, clinical trial using computerized diaries. Obstetrics and Gynecology 2002;100(3):435‐44. - PubMed
-
- Cohen L, Soares C, Yonkers K, et al. Paroxetine controlled release (CR) is effective in the treatment of premenstrual dysphoric disorder (PMDD): Results from a randomized placebo controlled trial. 15th Annual US Psychiatric and Mental Health Congress. October 28‐ November 3rd. Las Vegas, Nevada. 2002:Abstract 39.
-
- Judge R, Brown E, Miner C, Dillon J. Intermittent fluoxetine dosing in premenstrual dysphoric syndrome. World Journal of Biological Psychiatry 2001;2 Suppl:204.
-
- Miner C, Cohen LS, O'Brien PMS, Davis S, Brown E, Jacobson J. Predictors of response to luteal phase fluoxetine treatment of PMDD. The International Journal of Neuropsychopharmacology 2002;5 Suppl:87.
Cohen 2004 {published data only}
-
- Cohen L, Soares C, Yonkers K, Bellew K, Bridges I, Heller V. Paroxetine controlled release is effective in treating premenstrual dysphoric disorder. Obstetrics and Gynecology 2003;101 Suppl 4:111.
-
- Cohen L, Soares C, Yonkers K, Bellew K, Bridges I, Steiner M. Paroxetine controlled release for premenstrual dysphoric disorder: A double blind, placebo controlled trial. Psychosomatic Medicine 2004;66:707‐13. - PubMed
-
- GlaxoSmithKline. A Double‐Blind, Placebo‐Controlled, Three‐Arm Fixed‐Dose Study of Paroxetine CR Continuous Treatment (12.5 mg/day and 25 mg/day) for Premenstrual Dysphoric Disorder. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2F677&study... Accessed 16 August 2012.
-
- GlaxoSmithKline NZ. Phone call from a representative of GlaxoSmithKline New Zealand (in response to an email sent by J Marjoribanks) confirming that 29060/677 (Cohen 2004) and 29060/689 (Pearlstein 2005) were separate studies July 2012.
-
- Pearlstein 2012. Email to Jane Marjoribanks from Teri Pearlstein advising that participants in Pearlstein 2005 and Cohen 2004 did not, to her knowledge, overlap. 22.6.12.
Crnobaric 1998 {published data only}
-
- Crnobaric C, Jasovic‐Gasic M, Milanovanic S, Miljevic C. Treatment of premenstrual dysphoric disorder with fluoxetine during the luteal phase. 9th Congress of the Association of European Psychiatrists. Copenhagen, Denmark, 20‐24 September 1998.
Eriksson 1995 {published data only}
-
- Eriksson E, Hedberg MA, Andersch B, Sunblad C. The serotonin reuptake inhibitor paroxetin is superior to the noradrenaline reuptake inhibitor maprotiline in the treatment of premenstrual syndrome. Neuropsychopharmacology 1995;12:167‐76. - PubMed
Eriksson 2008 {published data only}
-
- Eriksson E, Ekman A, Sinclair S, Sorvik K, Ysandeer C, Mattson U‐B, Nissbrandt H. Escitalopram administered in the luteal phase exerts a marked and dose‐dependent effect in premenstrual dysphoric disorder. Journal of Clinical Psychopharmacology 2008;28(2):195‐202. - PubMed
Freeman 1999 {published data only}
-
- Freeman EW, Rickels K, Sondheimer S, Polansky M. Differential response to antidepressants in women with premenstrual syndrome. Archives of General Psychiatry 1999;56:932‐9. - PubMed
Freeman 2004 {published data only}
-
- Freeman E, Rickels K, Sondheimer S, Polansky M, Xiao S. Continuous or intermittent dosing with sertraline for patients with severe premenstrual syndrome or premenstrual dysphoric disorder. American Journal of Psychiatry 2004;161(2):343‐51. - PubMed
Freeman 2010 {unpublished data only}
-
- Freeman E. Escitalopram for Premenstrual Syndrome (PMS) in Teens: A Pilot Study NCT00523705. http://clinicaltrials.gov/ct2/show/NCT00523705.
Glaxo 1996 {unpublished data only}
-
- GlaxoSmithKline. A Double‐Blind, Placebo Controlled Study to Investigate the Efficacy, Safety and Tolerability of Paroxetine in Patients with Premenstrual Dysphoric Disorder. [Study No PAR 29060.400]. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2F400&study... Accessed 16 August 2012.
Glaxo 1996a {unpublished data only}
-
- GlaxoSmithKline. A Multicentre, Double‐Blind, Placebo‐Controlled Study to Investigate the Effects of Paroxetine (5mg OD Vs 10mg OD Vs 20mg OD) in Patients with Premenstrual Dysphoric Disorder (PMDD) [Study No PAR 29060.427]. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2F427&study... Accessed 16 August 2012.
Glaxo 2001 {unpublished data only}
-
- GlaxoSmithKline. A Double‐Blind, Placebo‐Controlled, 3‐Arm, Fixed‐Dose Study of Paroxetine CR Continuous Treatment (12.5 mg/day and 25 mg/day) for Premenstrual Dysphoric Disorder [Study No 29060/688]. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2F688&study... Accessed 16 August 2012.
Halbreich 1997 {published data only}
-
- Halbreich U, Smoller JW. Intermittent luteal phase sertraline treatment of dysphoric premenstrual syndrome. Journal of Clinical Psychiatry 1997;58:399‐402. - PubMed
Halbreich 2002 {published data only}
-
- Halbreich U, Bergeron R, Freeman E, Stout A, Cohen L. Intermittent (luteal phase) dosing of sertraline effective in PMDD. International Journal of Neuropsychopharmacology 2000;3 Suppl 1:248.
-
- Halbreich U, Bergeron R, Stout A, Freeman E, Yonkers K, Pearlstein T, et al. Dosing of sertraline is effective in premenstrual dysphoric disorder. 155th Annual Meeting of the American Psychiatric Association. 13th‐18th May 2000.
-
- Halbreich U, Bergeron R, Yonkers K, Freeman E, Stout A, Cohen L. Efficacy of intermittent, luteal phase sertraline treatment of premenstrual dysphoric disorder. Obstetrics and Gynecology 2002;100(6):1219‐29. - PubMed
-
- Pearlstein T, Yonkers K, Fayyad R, Gillespie J. Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. Journal of Affective Disorders 2005;85:275‐82. - PubMed
Jermain 1999 {published data only}
-
- Jermain DM, Preece CK, Sykes RL, Kuehl TJ, Sulak PJ. Luteal phase sertraline for the treatment of premenstrual dysphoric disorder. Archives of Family Medicine 1999;8:328‐32. - PubMed
Kornstein 2006 {published data only}
-
- Kornstein S, Gillespie J. Double blind placebo controlled study of sertraline in premenstrual syndrome. 155th Annual Meeting of the American Psychiatric Association. May 18‐23, Philadelphia PA. 2002:NR248.
-
- Kornstein S, Pearlstein T, Fayyad R, Farfel G, Gillespie J. Low dose sertraline in the treatment of moderate to severe premenstrual syndrome: Efficacy of 3 dosing strategies. Journal of Clinical Psychiatry 2006;67(10):1624‐32. - PubMed
Landen 2007 {published data only}
-
- Bellew KM, Landen M, Hunter B, McCafferty JP. Social functioning improves with paroxetine treatment. 155th Annual Meeting of the American Psychiatric Association. May 18‐23, Philadelphia. 2002:NR282.
-
- GlaxoSmithKline. A Placebo‐Controlled Study to Investigate the Efficacy of Intermittent and Continuous Treatment With Paroxetine in Patients With Premenstrual Dysphoric Disorder (PMDD). http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2F658&study... Accessed 16 August 2012.
-
- Landen M, Sorvik K, Ysander C, Allgulander C, Nissbrandt H, Gezelius B, et al. A placebo controlled trial exploring the efficacy of paroxetine for the treatment of premenstrual dysphoria. American Psychiatric Association, 155th annual meeting. May 18‐23. Philadelphia, PA., 2002.
-
- Landen M, Ysander K, Sorvik K, Nissbrandt H, Allgulander C, Hunter B, et al. A placebo controlled study of the efficacy of intermittent and continuous treatment with paroxetine for premenstrual dysphoric disorder (PMDD). European Neuropsychopharmacology 2001;11 Suppl 3:308‐9.
-
- Landen MSG. A Placebo‐Controlled Study to Investigate the Onset of Action of Paroxetine in Premenstrual Dysphoria. http://clinicaltrials.gov/ct2/show/NCT00516113 2007.
Menkes 1992 {published data only}
-
- Menkes DB, Taghavi E, Mason PA, Spears GF, Howard RC. Fluoxetine treatment of severe premenstrual syndrome. International Clinical Psychopharmacology 1993;8:95‐102. - PubMed
Miner 2002 {published data only}
-
- Miner C, Brown E, McCray S, Gonzales J, Wohlreich M. Weekly luteal phase dosing with enteric coated fluoxetine 90mg in premenstrual dysphoric disorder: A randomised, double blind, placebo controlled clinical study. Clinical Therapeutics 2002;24(3):417‐33. - PubMed
-
- Miner C, Cohen LS, O'Brien PMS, Davis S, Brown E, Jacobson J. Predictors of response to luteal phase fluoxetine treatment of PMDD. The International Journal of Neuropsychopharmacology 2002;5 Suppl:87.
Ozeren 1997 {published data only}
-
- Ozeren S, Corakci A, Yucesoy I, Mercan R, Erhan G. Fluoxetine in the treatment of premenstrual syndrome. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1997;73:167‐70. - PubMed
Pearlstein 1997 {published data only}
-
- Pearlstein TB, Stone AB, Lund SA, Scheft H, Zlotnick C, Brown WA. Comparison of fluoxetine, bupropion and placebo in the treatment of premenstrual dsyphoric disorder. Journal of Clinical Psychopharmacology 1997;17:261‐6. - PubMed
Pearlstein 2005 {published data only}
-
- GlaxoSmithKline. A Double‐Blind, Placebo‐Controlled, 3‐Arm, Fixed‐Dose Study of Paroxetine CR Continuous Treatment (12.5 mg/day and 25 mg/day) for Premenstrual Dysphoric Disorder. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2F689&study... Accessed 16 August 2012.
-
- GlaxoSmithKline NZ. Phone call from a representative of GlaxoSmithKline New Zealand (in response to an email sent by J. Marjoribanks) confirming that 29060/677 (Cohen 2004) and 29060/689 (Pearlstein 2005) were separate studies July 2012.
-
- Pearlstein 2012. Email to Jane Marjoribanks from Teri Pearlstein advising that participants in Pearlstein 2005 and Cohen 2004 did not, to her knowledge, overlap. 22.6.12.
-
- Pearlstein T, Bellew K, Endicott J, Steiner M. Paroxetine controlled release for premenstrual dysphoric disorder: remission analysis following a randomized, double blind, placebo controlled trial. 41st Annual Meeting of the American College of Neuropsychopharmacology (ACNP), San Juan, Puerto Rico. Dec 8‐12 2002.
Steiner 1995 {published data only}
-
- Dillon J, Steiner M, Judge R, Brown E. Fluoxetine improves social functioning in women with premenstrual dysphoria. International Journal of Gynecology and Obstetrics 2000;70 Suppl 3:99.
-
- Koke S, Steiner M, Judge R, Babcock S, Dillon J. Efficacy of fluoxetine in improving mood symptoms and social impairments in patients with PMDD. Psychosomatics 2001;42(2):178.
-
- Nilsson M Judge R, Brown E, Schuler C. Fluoxetine's efficacy in improving mood. physical and social impairment symptoms associated with PMDD. International Journal of Gynecology and Obstetrics 2000;Abstract Book 3:96.
-
- Steiner M, Babcock S, McCray SD, Romano S. Fluoxetine's efficacy in improving premenstrual dysphoric disorder. 152nd Annual Meeting of the American Psychiatric Association, Washington DC. USA. 15‐20th May 1999.
-
- Steiner M, Babcock S, Steinberg SI, Stewart DE, Carter D, Berger C. Fluoxetine's efficacy in improving physical symptoms associated with pdd: results from a multisite, randomized placebo controlled trial. 152nd Annual Meeting of the American Psychiatric Association. Washington DC USA. 15‐20 May 1999.
Steiner 2005 {published data only}
-
- Gee M, Bellew K, Holland F, Erp E, Perera P, McCafferty J. Luteal phase dosing of paroxetine controlled release is effective in treating PMDD. 156th Annual Meeting American Psychiatric Association, May 17‐22 San Francisco, CA. 2003:NR760.
-
- GlaxoSmithKline. A Double‐Blind, Placebo‐Controlled, 3‐Arm, Fixed‐Dose Study of Paroxetine CR Intermittent Dosing (12.5 mg and 25 mg) for Premenstrual Dysphoric Disorder [Study No 29060/717]. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2F717&study... Accessed 16 August 2012.
-
- Steiner M, Hirschberg AL, Bergeron R, Holland F, Gee MD, Erp E. Luteal phase dosing with paroxetine controlled release (CR) in the treatment of premenstrual dysphoric disorder. American Journal of Obstetrics and Gynecology 2005;193:352‐60. - PubMed
Steiner 2008 {published data only}
-
- GlaxoSmithKline. A randomized, double‐blind, placebo‐controlled trial of intermittent treatment with paroxetine 10mg/day and 20mg/day versus placebo in Canadian women with Premenstrual Dysphoric Disorder. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2F621&study... Accessed 16 August 2012.
-
- Hamilton Health Sciences Corporation. Luteal Phase Administration of Paroxetine for the Treatment of PMDD: A Randomized, Double‐Blind, Placebo‐Controlled Trial in Canadian Women NCT00620581. http://clinicaltrials.gov/ct2/show/NCT00620581 2008. - PubMed
-
- Steiner M, Ravindran AV, LeMedello J‐M, Carter D, Huang JO, Anonychuk AM, Simpson SD. Luteal phase administration of paroxetine for the treatment of premenstrual dysphoric disorder: a randomized, double‐blind, placebo‐controlled trial in Canadian women. Journal of Clinical Psychiatry 2008;69:991‐8. - PubMed
Stone 1991 {published data only}
-
- Stone AB, Pearlstein TB, Brown WA. Fluoxetine in the treatment of late luteal phase dysphoric disorder. Journal of Clinical Psychiatry 1991;52:290‐3. - PubMed
-
- Stone AB, Pearlstein TB, Brown WA. Fluoxetine in the treatment of premenstrual syndrome. Psychopharmacology Bulletin 1990;26:331‐5. - PubMed
-
- Yonkers KA, Halbreich U, Freeman E, Brown C, Pearlstein T. Sertraline in the treatment of premenstrual dysphoric disorder. Psychopharmacology Bulletin 1996;32(1):41‐6. - PubMed
Su 1997 {published data only}
-
- Su TP, Schmidt PJ, Danaceau MA, Tobin MB, Rosenstein RL, Murphy DL, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology 1997;16(5):346‐56. - PubMed
Wikander 1998 {published data only}
-
- Wikander I, Sunblad C, Andersch B, Dagnell I, Zylberstein D, Bengtsson F, et al. Citalopram in premenstrual dysphoria: Is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle?. Journal of Clinical Psychopharmacology 1998;18:390‐8. - PubMed
Wood 1992 {published data only}
-
- Wood SH, Mortola JF, Chan YF, Moossazadeh F, Yen SS. Treatment of premenstrual syndrome with fluoxetine: a double‐blind, placebo‐controlled, crossover study. Obstetrics and Gynecology 1992;80:339‐44. - PubMed
Yonkers 1997 {published data only}
-
- Yonkers KA, Halbriech U, Freeman E, Brown C, Endicott J, Frank E, et al. Symptomatic improvement of premenstrual dysphoric disorder with sertraline treatment. JAMA 1997;278(12):983‐8. - PubMed
Young 1998 {published data only}
-
- Young SA, Hurt PH, Benedeck DM, Howard RS. Treatment of PDD with sertraline during the luteal phase: a randomised, double‐blind, placebo controlled crossover trial. Journal of Clinical Psychiatry 1998;59:76‐80. - PubMed
-
- Young SA, Hurt PH, Benedek DM, Howard RS. Treatment of premenstrual dysphoric disorder with sertraline during the luteal phase: A randomized, double blind placebo controlled crossover trial. American Psychiatric Association 150th Annual Meeting, San Diego USA. 1997. - PubMed
-
- Young SA, Hurt PH, Benedek DM, Howard RS. Treatment of premenstrual dysphoric disorder with sertraline during the luteal phase: A randomized, double blind placebo controlled crossover trial. Journal of Clinical Psychiatry 1998;59:76‐80. - PubMed
References to studies excluded from this review
Alpay 2001 {published data only}
-
- Alpay F, Turhan N. Intermittant versus continuous sertraline therapy in the treatment of premenstrual dysphoric disorders. International Journal of Fertility 2001;46:228‐31. - PubMed
Brandenburg 1993 {published data only}
-
- Brandenburg S, Tuynman‐Qua H, Verheij R, Pepplinkhuizen L. Treatment of premenstrual syndrome with fluoxetine: an open study. International Clinical Psychopharmacology 1993;8:315‐7. - PubMed
De la Gandara 1997 {published data only}
-
- Gandara Martin JJ. Trastorno disforico premenstrual: Tratamiento a largo plazo con fluoxetina y discontinuacion [Premenstrual dysphoric disorder:long term treatment with fluoxetine and discontinuation]. Actas Luso‐Espanolas de Neurologia, Psiquiatria y Ciencias Afines 1997;25:235‐42. - PubMed
Diegoli 1998 {published data only}
-
- Diegoli M, DaFonseca A, Diegoli CA, Pinotti JA. A double blind trial of four medications to treat severe premenstrual syndrome. International Journal of Gynecology and Obstetrics 1998;62:63‐7. - PubMed
Elks 1993 {published data only}
-
- Elks ML. Open trial of fluoxetine therapy for premenstrual syndrome. Southern Medical Journal 1993;86:503‐7. - PubMed
Flores Ramos 2003 {published data only}
-
- Fores Ramos M, Ontiveros Uribe M, Cortes Sotres J. Comparison between continuous and intermittent treatment with citalopram..... [Comparacion entre el tratamiento continuo y el intermitente con citalopram para el trastorno disforico premenstrual]. Salud Mental 2003;26(003):37‐45.
Freeman 1996 {published data only}
-
- Freeman EW, Rickels K, Sondheimer SJ. Comparison of serotonergic and noradrenergic antidepressant medications in treatment of premenstrual syndrome (PMS). XXIst Collegium Internationale Neuro‐psychopharmacologicum, Glasgow, Scotland. 12‐16th July 1998.
-
- Freeman EW, Rickels K, Sondheimer SJ. Sertraline versus desipramine in PMS treatment. 151st Annual Meeting of the American Psychiatric Association, Toronto, Canada. 30th May‐4th June 1998, issue 118C.
-
- Freeman EW, Rickels K, Sondheimer SJ, Wittmaack FM. Sertraline versus desipramine in the treatment of premenstrual syndrome: an open label trial. Journal of Clinical Psychiatry 1996;57:7‐11. - PubMed
Freeman 1999b {published data only}
-
- Freeman EW, Rickels K, Arredono F, Lee‐Chuan K, Pollack S, Sondheimer S. Full or half cycle treatment of severe premenstrual syndrome with a serotonergic antidepressant. Journal of Cliniical Psychopharmacology 1999;19(1):3‐9. - PubMed
Freeman 2000 {published data only}
-
- Freeman EW, Sondheimer SJ, Polansky M, Garcia‐Espagna B. Predictors of response to sertraline treatment of severe premenstrual syndromes. Journal of Clinical Psychiatry 2000;61(8):579‐84. - PubMed
Freeman 2002 {published data only}
-
- Freeman E, Jabara S, Sondheimer S, Auletto R. Citalopram in PMS patients with prior SSRI treatment failure: A preliminary study. Journal of Womens Health & Gender‐based Medicine 2002;11(5):459‐64. - PubMed
Freeman 2005 {published data only}
-
- Freeman E, Sondheimer S, Sammel M, Ferdousi T, Lin H. A preliminary study of luteal phase versus symptom‐onset dosing with escitalopram for premenstrual dysphoric disorder. Journal of Clinical Psychiatry 2005;66(6):769‐73. - PubMed
Glaxo 2002 {unpublished data only}
-
- GlaxoSmithKline. A 3‐Month, Double‐blind, Placebo‐controlled, Fixed‐dose, Extension Study of Paroxetine CR (12.5 mg and 25 mg/day) Continuous Treatment for PMDD Patients Completing Studies 29060/677, 688 or 689 [Study no 29060/711]. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2F711&study... Accessed 16 August 2012.
Landen 2009 {published data only}
-
- Landen M, Erlandsson H, Bengtsson F, Andersch B, Eriksson E. Short onset of action of a serotonin reuptake inhibitor when used to reduce premenstrual irritability. Neuropsychopharmacology 2009;34:585‐92. - PubMed
Miller 2008 {published data only}
-
- Miller MN, Newell CL, Miller BE, Frizzell PG, Kayser RA, Ferslew KE. Variable dosing of sertraline for premenstrual exacerbation of depression: a pilot study. Journal of Women's Health 2008;17(6):993‐7. - PubMed
Pearlstein 1994 {published data only}
-
- Pearlstein TB, Stone AB. Long term fluoxetine treatment of late luteal phase dysphoric disorder. Journal of Clinical Psychiatry 1994;55:332‐5. - PubMed
Pearlstein 2000 {published data only}
-
- Pearlstein T, Haskett R, Stout A, Frank E, Endicott J. Sertraline improves psychosocial functioning in premenstrual dysphoric disorder. 151st Annual Meeting of the American Psychiatric Association, 30th May‐ 4th June Toronto, Canada. 1998.
-
- Pearlstein TB, Halbreich U, Batzar ED, Brown CS, Endicott J, Frank E, et al. Psychosocial functioning in women with premenstrual dysphoric disorder before and after treatment with sertraline or placebo. Journal of Clinical Psychiatry 2000;61(2):101‐9. - PubMed
Rickels 1990 {published data only}
-
- Rickels K. Fluoxetine in the treatment of PMS. Current Therapeutic Research 1990;48:161‐8.
Steiner 1997 {published data only}
-
- Steiner M, Korzekwa MI, Lamont J, Wilkins A. Intermittent fluoxetine dosing in the treatment of women with premenstrual dysphoria. Psychopharmacology Bulletin 1997;33:771‐4. - PubMed
Sundblad 1992 {published data only}
-
- Sundblad C, Modigh K, Andersch B, Eriksson E. Clomipramine effectively reduces irritability and dysphoria: a placebo controlled trial. Acta Psychiatrica Scandinavia 1992;85:39‐47. - PubMed
Sundblad 1993 {published data only}
-
- Sundblad C, Hedberg M, Eriksson E. Clomipramine administered during the luteal phase reduces the symptoms of premenstrual syndrome: A placebo controlled trial. Neuropsychopharmacology 1993;9(2):133‐45. - PubMed
Sundblad 1997 {published data only}
-
- Sundblad C Wikander I, Andersch B, Eriksson E. A naturalistic study of paroxetine in premenstrual syndrome: efficacy and side‐effects during ten cycles of treatment. European Neuropsychopharmacology 1997;7(3):201‐6. - PubMed
Veeninga 1990 {published data only}
-
- Veeninga AT, Westenberg HG, Weusten JT. Fluvoxamine in the treatment of menstrually related disorders. Psychopharmacology 1990;102:414‐6. - PubMed
Wu 2008 {published data only}
-
- Wu K‐Y, Liu C‐Y, Hsiao M‐C. Six‐month paroxetine treatment of premenstrual dysphoric disorder: continuous versus intermittent treatment protocols. Psychiatry and Clinical Neurosciences 2008;62:109‐14. - PubMed
Yonkers 1996 {published data only}
-
- Yonkers KA, Gullion C, Williams A, Novak K, Rush AJ. Paroxetine as a treatment for premenstrual dysphoric disorder. Journal of Clinical Psychopharmacology 1996;16(1):3‐8. - PubMed
Yonkers 2002 {unpublished data only}
-
- Yonkers KA. Antidepressant Treatment for Premenstrual Syndrome and Premenstrual Dysphoric Disorder. http://clinicaltrials.gov/ct2/show/NCT00048854.
Yonkers 2006 {published data only}
-
- Yonkers K, Holthausen G, Poschman K, Howell H. Symptom‐onset treatment for women with premenstrual dysphoric disorder. Journal of Clinical Psychopharmacology 2006;26(2):198‐202. - PubMed
References to ongoing studies
Yonkers 2007 {unpublished data only}
-
- Yonkers KA, Altemus M, Kornstein S. Evaluating the Effectiveness of Sertraline in Treating Women With Premenstrual Dysphoric Disorder. http://www.clinicaltrials.gov/ct2/show/NCT00536198 2007.
Yonkers 2010 {unpublished data only}
-
- Yonkers KA. Comparison of Fluoxetine, Calcium and Placebo for the Treatment of Moderate to Severe Premenstrual Syndrome (PMS). http://clinicaltrials.gov/ct2/show/NCT00965562 2010.
Additional references
ACOG 2000
-
- American College of Obstetricians and Gynecologists. Premenstrual syndrome. ACOG PracticeBulletin No. 15. Washington, DC: ACOG 2000.
APA 2000
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC:American Psychiatric Association; 2000. p. 774. Diagnostic and statistical manual of mental disorders. 4th Edition. Washington, DC: American Psychiatric Association, 2000:774.
Baker 2012
-
- Baker LJ, O'Brien PMS. Premenstrual syndrome (PMS): A peri‐menopausal perspective. Maturitas 72 2012;72:121‐5. - PubMed
Cohen 1988a
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences (2nd edition). Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988.
Epperson 2012
Ford 2012
Freeman 2009
Freeman 2012
Glaxo 2012
-
- GlaxoSmithKline Government Affairs, Public Policy, Patient Advocacy. Public Disclosure of Clinical Research. Downloaded from: http://www.google.co.nz/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0CD....
Halbreich 2008
-
- Halbreich U. Selective serotonin reuptake inhibitors and initial oral contraceptives for the treatment of PMDD: effective but not enough. CNS Spectrum 2008;13(7):566,569‐72. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011 ]. Available from www.cochrane‐handbook.org.
Ismail 2006
-
- Ismail KMK, Crome IB, O'Brien PMS. In: Higham J editor(s). Psychological disorders in obstetrics and gynaecology for the MRCOG and beyond. London: The Royal College of Obstetricicians and Gynaecologists, 2006:37.
Jing 2009
Kleinstauber 2012
-
- Kleinstauber M, Witthoft M, Hiller W. Cognitive‐behavioral and pharmacological interventions for premenstrual syndrome or premenstrual dysphoric disorder: a meta‐analysis. Journal of Clinical Psychology in Medical Settings 2012;19:308‐19. - PubMed
Lopez 2012
Magos 1986
-
- Magos AL, Brincat M, Studd JW. Trend analysis of the symptoms of 150 women with a history of th premenstrual syndrome. American Journal of Obstetrics and Gynecology 1986;155(2):277‐82. - PubMed
Micromedex 2013
-
- DRUGDEX® Evaluations. Micromedex.2 database Accessed 2.5.13 via University of Auckland library.
Naheed 2013
O'Brien 2011
Pearlstein 2002
-
- Pearlstein T. Selective serotonin reuptake inhibitors for premenstrual dysphoric disorder. Drugs 2002;62(13):1869‐85. - PubMed
Pearlstein 2007
-
- Pearlstein T. Prevalence, impact, on morbidity and burden of disease. In: O’Brien PMS, Rapkin A, Schmidt P editor(s). The premenstrual syndromes: PMS and PMDD. London: Informa Healthcare, 2007:37‐47.
Plouffe 1993
-
- Plouffe L, Stewart K, Craft KS, Maddox MS, Rausch MD. Diagnostic and treatment results from a south‐eastern academic center‐based premenstrual syndrome clinic: The first year. Americal Journal of Obstetrics and Gynecology 1993;169(2):295‐307. - PubMed
Rapkin 2008
-
- Rapkin AJ, Winer SA. The pharmacologic management of premenstrual dysphoric disorder. Expert Opinion in Pharmacotherapy 2008;9(3):429‐45. - PubMed
Shah 2008
Shaw 2003
-
- Shaw S, Wyatt K, Campbell J, Ernst E, Thompson‐Coon J. Vitex agnus castus for premenstrual syndrome. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004632] - DOI
Yu 2005
-
- Yu J, Liu B, Liu Z, Welch V, Wu T, Clarke J, Smith CA. Acupuncture for premenstrual syndrome. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005290] - DOI
References to other published versions of this review
Dimmock 2000
-
- Dimmock PW, Wyatt KM, Jones PW, O'Brien PMS. Efficacy of selective serotonin‐reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet 2000;356:1131‐6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


