Dynamic coding for cognitive control in prefrontal cortex
- PMID: 23562541
- PMCID: PMC3898895
- DOI: 10.1016/j.neuron.2013.01.039
Dynamic coding for cognitive control in prefrontal cortex
Abstract
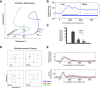
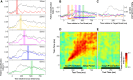
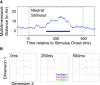
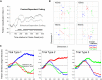
Cognitive flexibility is fundamental to adaptive intelligent behavior. Prefrontal cortex has long been associated with flexible cognitive function, but the neurophysiological principles that enable prefrontal cells to adapt their response properties according to context-dependent rules remain poorly understood. Here, we use time-resolved population-level neural pattern analyses to explore how context is encoded and maintained in primate prefrontal cortex and used in flexible decision making. We show that an instruction cue triggers a rapid series of state transitions before settling into a stable low-activity state. The postcue state is differentially tuned according to the current task-relevant rule. During decision making, the response to a choice stimulus is characterized by an initial stimulus-specific population response but evolves to different final decision-related states depending on the current rule. These results demonstrate how neural tuning profiles in prefrontal cortex adapt to accommodate changes in behavioral context. Highly flexible tuning could be mediated via short-term synaptic plasticity.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Limber neurons for a nimble mind.Neuron. 2013 Apr 24;78(2):211-3. doi: 10.1016/j.neuron.2013.04.007. Neuron. 2013. PMID: 23622059 Free PMC article.
Similar articles
-
Representation of Behavioral Tactics and Tactics-Action Transformation in the Primate Medial Prefrontal Cortex.J Neurosci. 2016 Jun 1;36(22):5974-87. doi: 10.1523/JNEUROSCI.4572-15.2016. J Neurosci. 2016. PMID: 27251619 Free PMC article.
-
Neural Correlates of Strategy Switching in the Macaque Orbital Prefrontal Cortex.J Neurosci. 2020 Apr 8;40(15):3025-3034. doi: 10.1523/JNEUROSCI.1969-19.2020. Epub 2020 Feb 25. J Neurosci. 2020. PMID: 32098903 Free PMC article.
-
Monkey Prefrontal Neurons Reflect Logical Operations for Cognitive Control in a Variant of the AX Continuous Performance Task (AX-CPT).J Neurosci. 2016 Apr 6;36(14):4067-79. doi: 10.1523/JNEUROSCI.3578-15.2016. J Neurosci. 2016. PMID: 27053213 Free PMC article.
-
Integration of cognitive and motivational context information in the primate prefrontal cortex.Cereb Cortex. 2007 Sep;17 Suppl 1:i101-9. doi: 10.1093/cercor/bhm067. Cereb Cortex. 2007. PMID: 17725993 Review.
-
Reward-dependent learning in neuronal networks for planning and decision making.Prog Brain Res. 2000;126:217-29. doi: 10.1016/S0079-6123(00)26016-0. Prog Brain Res. 2000. PMID: 11105649 Review.
Cited by
-
A working memory model based on recurrent neural networks using reinforcement learning.Cogn Neurodyn. 2024 Oct;18(5):3031-3058. doi: 10.1007/s11571-024-10137-6. Epub 2024 Jun 13. Cogn Neurodyn. 2024. PMID: 39555285
-
Cognitive networks interactions through communication subspaces in large-scale models of the neocortex.bioRxiv [Preprint]. 2024 Dec 6:2024.11.01.621513. doi: 10.1101/2024.11.01.621513. bioRxiv. 2024. PMID: 39554020 Free PMC article. Preprint.
-
Is Cognitive Flexibility Equivalent to Shifting? Investigating Cognitive Flexibility in Multiple Domains.J Cogn. 2024 Oct 10;7(1):73. doi: 10.5334/joc.403. eCollection 2024. J Cogn. 2024. PMID: 39398223 Free PMC article.
-
Rational inattention in neural coding for economic choice.bioRxiv [Preprint]. 2024 Sep 23:2024.09.20.614193. doi: 10.1101/2024.09.20.614193. bioRxiv. 2024. PMID: 39386501 Free PMC article. Preprint.
-
A transient high-dimensional geometry affords stable conjunctive subspaces for efficient action selection.Nat Commun. 2024 Oct 1;15(1):8513. doi: 10.1038/s41467-024-52777-6. Nat Commun. 2024. PMID: 39353961 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


