Selective degeneration of dopaminergic neurons by MPP(+) and its rescue by D2 autoreceptors in Drosophila primary culture
- PMID: 23452092
- PMCID: PMC3737274
- DOI: 10.1111/jnc.12228
Selective degeneration of dopaminergic neurons by MPP(+) and its rescue by D2 autoreceptors in Drosophila primary culture
Abstract
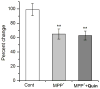
Drosophila melanogaster is widely used to study genetic factors causing Parkinson's disease (PD) largely because of the use of sophisticated genetic approaches and the presence of a high conservation of gene sequence/function between Drosophila and mammals. However, in Drosophila, little has been done to study the environmental factors which cause over 90% of PD cases. We used Drosophila primary neuronal culture to study degenerative effects of a well-known PD toxin MPP(+) . Dopaminergic (DA) neurons were selectively degenerated by MPP(+) , whereas cholinergic and GABAergic neurons were not affected. This DA neuronal loss was because of post-mitotic degeneration, not by inhibition of DA neuronal differentiation. We also found that MPP(+) -mediated neurodegeneration was rescued by D2 agonists quinpirole and bromocriptine. This rescue was through activation of Drosophila D2 receptor DD2R, as D2 agonists failed to rescue MPP(+) -toxicity in neuronal cultures prepared from both a DD2R deficiency line and a transgenic line pan-neuronally expressing DD2R RNAi. Furthermore, DD2R autoreceptors in DA neurons played a critical role in the rescue. When DD2R RNAi was expressed only in DA neurons, MPP(+) toxicity was not rescued by D2 agonists. Our study also showed that rescue of DA neurodegeneration by Drosophila DD2R activation was mediated through suppression of action potentials in DA neurons.
Keywords: D2 agonist; DD2R; Drosophila melanogaster; Parkinson's disease; primary neuronal culture.
© 2013 International Society for Neurochemistry.
Conflict of interest statement
Figures



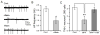
Similar articles
-
Role of Drosophila calcium channel cacophony in dopaminergic neurodegeneration and neuroprotection.Neurosci Lett. 2015 Jan 1;584:342-6. doi: 10.1016/j.neulet.2014.11.004. Epub 2014 Nov 6. Neurosci Lett. 2015. PMID: 25445363
-
Locomotor activity is regulated by D2-like receptors in Drosophila: an anatomic and functional analysis.Dev Neurobiol. 2007 Feb 15;67(3):378-93. doi: 10.1002/dneu.20355. Dev Neurobiol. 2007. PMID: 17443795
-
Dopamine D2 receptor as a cellular component controlling nocturnal hyperactivities in Drosophila melanogaster.Chronobiol Int. 2013 May;30(4):443-59. doi: 10.3109/07420528.2012.741169. Epub 2013 Jan 3. Chronobiol Int. 2013. PMID: 23286280
-
The role of D2-autoreceptors in regulating dopamine neuron activity and transmission.Neuroscience. 2014 Dec 12;282:13-22. doi: 10.1016/j.neuroscience.2014.01.025. Epub 2014 Jan 23. Neuroscience. 2014. PMID: 24463000 Free PMC article. Review.
-
Big Lessons from Tiny Flies: Drosophila melanogaster as a Model to Explore Dysfunction of Dopaminergic and Serotonergic Neurotransmitter Systems.Int J Mol Sci. 2018 Jun 16;19(6):1788. doi: 10.3390/ijms19061788. Int J Mol Sci. 2018. PMID: 29914172 Free PMC article. Review.
Cited by
-
Endogenous tau released from human ReNCell VM cultures by neuronal activity is phosphorylated at multiple sites.bioRxiv [Preprint]. 2024 Jun 2:2024.06.02.597022. doi: 10.1101/2024.06.02.597022. bioRxiv. 2024. PMID: 38854111 Free PMC article. Preprint.
-
Neuroprotective Activity of Enantiomers of Salsolinol and N-Methyl-(R)-salsolinol: In Vitro and In Silico Studies.ACS Omega. 2023 Oct 5;8(41):38566-38576. doi: 10.1021/acsomega.3c05527. eCollection 2023 Oct 17. ACS Omega. 2023. PMID: 37867702 Free PMC article.
-
Outlook of PINK1/Parkin signaling in molecular etiology of Parkinson's disease, with insights into Pink1 knockout models.Zool Res. 2023 May 18;44(3):559-576. doi: 10.24272/j.issn.2095-8137.2022.406. Zool Res. 2023. PMID: 37161651 Free PMC article. Review.
-
Quinpirole ameliorates nigral dopaminergic neuron damage in Parkinson's disease mouse model through activating GHS-R1a/D2R heterodimers.Acta Pharmacol Sin. 2023 Aug;44(8):1564-1575. doi: 10.1038/s41401-023-01063-0. Epub 2023 Mar 10. Acta Pharmacol Sin. 2023. PMID: 36899113 Free PMC article.
-
Regulation and modulation of biogenic amine neurotransmission in Drosophila and Caenorhabditis elegans.Front Physiol. 2023 Feb 16;14:970405. doi: 10.3389/fphys.2023.970405. eCollection 2023. Front Physiol. 2023. PMID: 36875033 Free PMC article. Review.
References
-
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295(5556):865–8. - PubMed
-
- Barolo S, Castro B, Posakony JW. New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. Biotechniques. 2004;36:436–42. - PubMed
-
- Bilen J, Bonini NM. Drosophila as a model for human neurodegenerative disease. Annu Rev Genet. 2005;39:153–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases


