Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior
- PMID: 23401542
- PMCID: PMC3587265
- DOI: 10.1073/pnas.1219731110
Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior
Abstract
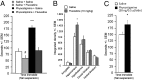
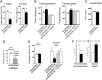
Symptoms of depression can be induced in humans through blockade of acetylcholinesterase (AChE) whereas antidepressant-like effects can be produced in animal models and some clinical trials by limiting activity of acetylcholine (ACh) receptors. Thus, ACh signaling could contribute to the etiology of mood regulation. To test this hypothesis, we administered the AChE inhibitor physostigmine to mice and demonstrated an increase in anxiety- and depression-like behaviors that was reversed by administration of nicotinic or muscarinic antagonists. The behavioral effects of physostigmine were also reversed by administration of the selective serotonin reuptake inhibitor fluoxetine. Administration of fluoxetine also increased AChE activity throughout the brain, with the greatest change in the hippocampus. To determine whether cholinergic signaling in the hippocampus could contribute to the systemic effects of cholinergic drugs, we infused physostigmine or virally delivered shRNAs targeting AChE into the hippocampus. Both pharmacological and molecular genetic decreases in hippocampal AChE activity increased anxiety- and depression-like behaviors and decreased resilience to repeated stress in a social defeat paradigm. The behavioral changes due to shRNA-mediated knockdown of AChE were rescued by coinfusion of an shRNA-resistant AChE transgene into the hippocampus and reversed by systemic administration of fluoxetine. These data demonstrate that ACh signaling in the hippocampus promotes behaviors related to anxiety and depression. The sensitivity of these effects to fluoxetine suggests that shRNA-mediated knockdown of hippocampal AChE represents a model for anxiety- and depression-like phenotypes. Furthermore, abnormalities in the cholinergic system may be critical for the etiology of mood disorders and could represent an endophenotype of depression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Hippocampal α7 nicotinic ACh receptors contribute to modulation of depression-like behaviour in C57BL/6J mice.Br J Pharmacol. 2018 Jun;175(11):1903-1914. doi: 10.1111/bph.13769. Epub 2017 Apr 8. Br J Pharmacol. 2018. PMID: 28264149 Free PMC article.
-
SKF83959 produces antidepressant effects in a chronic social defeat stress model of depression through BDNF-TrkB pathway.Int J Neuropsychopharmacol. 2014 Dec 7;18(6):pyu096. doi: 10.1093/ijnp/pyu096. Int J Neuropsychopharmacol. 2014. PMID: 25522427 Free PMC article.
-
Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression.Neurosci Lett. 2015 Jun 15;597:121-6. doi: 10.1016/j.neulet.2015.04.036. Epub 2015 Apr 24. Neurosci Lett. 2015. PMID: 25916883
-
[Anti-apoptotic protein enhances resilience to psychoemotional effects of stress].Usp Fiziol Nauk. 2013 Apr-Jun;44(2):3-13. Usp Fiziol Nauk. 2013. PMID: 23789349 Review. Russian.
-
Acrylamide Neurotoxicity as a Possible Factor Responsible for Inflammation in the Cholinergic Nervous System.Int J Mol Sci. 2022 Feb 12;23(4):2030. doi: 10.3390/ijms23042030. Int J Mol Sci. 2022. PMID: 35216144 Free PMC article. Review.
Cited by
-
Fetal programming by the parental microbiome of offspring behavior, and DNA methylation and gene expression within the hippocampus.bioRxiv [Preprint]. 2024 Oct 22:2024.04.12.589237. doi: 10.1101/2024.04.12.589237. bioRxiv. 2024. PMID: 39484583 Free PMC article. Preprint.
-
Medial prefrontal cortex acetylcholine signaling mediates the ability to learn an active avoidance response following learned helplessness training.Neuropsychopharmacology. 2024 Oct 3. doi: 10.1038/s41386-024-02003-0. Online ahead of print. Neuropsychopharmacology. 2024. PMID: 39362985
-
A neural circuit for alcohol withdrawal-induced hyperalgesia in a nondependent state.Sci Adv. 2024 Sep 27;10(39):eadp8636. doi: 10.1126/sciadv.adp8636. Epub 2024 Sep 27. Sci Adv. 2024. PMID: 39331713 Free PMC article.
-
Integrated Long Noncoding RNA and Messenger RNA Expression Analysis Identifies Molecules Specifically Associated With Resiliency and Susceptibility to Depression and Antidepressant Response.Biol Psychiatry Glob Open Sci. 2024 Jul 20;4(6):100365. doi: 10.1016/j.bpsgos.2024.100365. eCollection 2024 Nov. Biol Psychiatry Glob Open Sci. 2024. PMID: 39257693 Free PMC article.
-
Repeated exposure to multiple concurrent stressors alters visual processing in the adult posterior parietal cortex.Neurobiol Stress. 2024 Jul 5;31:100660. doi: 10.1016/j.ynstr.2024.100660. eCollection 2024 Jul. Neurobiol Stress. 2024. PMID: 39100726 Free PMC article.
References
-
- Janowsky DS. Serendipity strikes again: Scopolamine as an antidepressant agent in bipolar depressed patients. Curr Psychiatry Rep. 2011;13(6):443–445. - PubMed
-
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases


