Pharmacokinetics and pharmacodynamics of the proton pump inhibitors
- PMID: 23350044
- PMCID: PMC3548122
- DOI: 10.5056/jnm.2013.19.1.25
Pharmacokinetics and pharmacodynamics of the proton pump inhibitors
Abstract
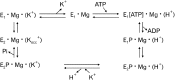
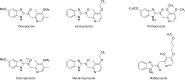
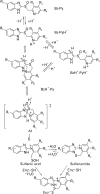
Proton pump inhibitor (PPI) is a prodrug which is activated by acid. Activated PPI binds covalently to the gastric H(+), K(+)-ATPase via disulfide bond. Cys813 is the primary site responsible for the inhibition of acid pump enzyme, where PPIs bind. Omeprazole was the first PPI introduced in market, followed by pantoprazole, lansoprazole and rabeprazole. Though these PPIs share the core structures benzimidazole and pyridine, their pharmacokinetics and pharmacodynamics are a little different. Several factors must be considered in understanding the pharmacodynamics of PPIs, including: accumulation of PPI in the parietal cell, the proportion of the pump enzyme located at the canaliculus, de novo synthesis of new pump enzyme, metabolism of PPI, amounts of covalent binding of PPI in the parietal cell, and the stability of PPI binding. PPIs have about 1hour of elimination half-life. Area under the plasmic concentration curve and the intragastric pH profile are very good indicators for evaluating PPI efficacy. Though CYP2C19 and CYP3A4 polymorphism are major components of PPI metabolism, the pharmacokinetics and pharmacodynamics of racemic mixture of PPIs depend on the CYP2C19 genotype status. S-omeprazole is relatively insensitive to CYP2C19, so better control of the intragastric pH is achieved. Similarly, R-lansoprazole was developed in order to increase the drug activity. Delayed-release formulation resulted in a longer duration of effective concentration of R-lansoprazole in blood, in addition to metabolic advantage. Thus, dexlansoprazole showed best control of the intragastric pH among the present PPIs. Overall, PPIs made significant progress in the management of acid-related diseases and improved health-related quality of life.
Keywords: Area under the plasmic concentration curve; Gastric acid; Gastric endogenous activator protein, mammal; Hydrogen potassium ATPase; Pharmacokinetics; Pharmacology; Proton pump inhibitors.
Conflict of interest statement
Conflicts of interest: None.
Figures

Similar articles
-
Pharmacokinetics of proton pump inhibitors in children.Clin Pharmacokinet. 2005;44(5):441-66. doi: 10.2165/00003088-200544050-00001. Clin Pharmacokinet. 2005. PMID: 15871633 Review.
-
Effects of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of proton pump inhibitors.Pharmacol Res. 2020 Feb;152:104606. doi: 10.1016/j.phrs.2019.104606. Epub 2019 Dec 14. Pharmacol Res. 2020. PMID: 31846760 Review.
-
Relative efficacies of gastric proton-pump inhibitors on a milligram basis: desired and undesired SH reactions. Impact of chirality.Scand J Gastroenterol Suppl. 2001;(234):3-9. doi: 10.1080/003655201753265389. Scand J Gastroenterol Suppl. 2001. PMID: 11768559
-
Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities.Drug Metab Dispos. 2004 Aug;32(8):821-7. doi: 10.1124/dmd.32.8.821. Drug Metab Dispos. 2004. PMID: 15258107
-
[New-generation proton pump inhibitors: progress in the treatment of peptic acid diseases?].Presse Med. 2004 Jun 19;33(11):746-54. doi: 10.1016/s0755-4982(04)98731-3. Presse Med. 2004. PMID: 15257232 Review. French.
Cited by
-
Proton pump inhibitor use and risk of stroke: A systematic review and meta-analysis.Pak J Med Sci. 2024 Nov;40(10):2432-2440. doi: 10.12669/pjms.40.10.10409. Pak J Med Sci. 2024. PMID: 39554645 Free PMC article. Review.
-
Comparative Efficacy of Dexlansoprazole, Pantoprazole, Esomeprazole, and Rabeprazole in Achieving Optimal 24-Hour Intragastric pH Control: A Randomized Crossover Study Using Ambulatory pH Monitoring.Cureus. 2024 Oct 14;16(10):e71418. doi: 10.7759/cureus.71418. eCollection 2024 Oct. Cureus. 2024. PMID: 39539895 Free PMC article.
-
Proton-Pump Inhibitors and Fat Absorption in Cystic Fibrosis and Pancreatic Insufficiency: A Randomized Crossover Pilot Trial.Dig Dis Sci. 2024 Nov 13. doi: 10.1007/s10620-024-08728-8. Online ahead of print. Dig Dis Sci. 2024. PMID: 39537890
-
Altered Microbiome Promotes Pro-Inflammatory Pathways in Oesophago-Gastric Tumourigenesis.Cancers (Basel). 2024 Oct 9;16(19):3426. doi: 10.3390/cancers16193426. Cancers (Basel). 2024. PMID: 39410045 Free PMC article. Review.
-
Efficacy and safety of Vonoprazan-based treatment of Helicobacter pylori infection: a systematic review and network meta-analysis.BMC Infect Dis. 2024 Sep 11;24(1):953. doi: 10.1186/s12879-024-09885-x. BMC Infect Dis. 2024. PMID: 39261752 Free PMC article.
References
-
- Black JW, Duncan WA, Durant CJ, Ganellin CR, Parsons ME. Definition and antagonism of histamine H2-receptors. Nature. 1972;236:385–390. - PubMed
-
- Fellenius E, Berglindh T, Sachs G, et al. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+) ATPase. Nature. 1981;290:159–161. - PubMed
-
- Okabe S, Takeuchi K, Urushidani T, Takagi K. Effects of cimetidine, a histamine H2-receptor antagonist, on various experimental gastric and duodenal ulcers. Am J Dig Dis. 1977;22:677–684. - PubMed
-
- Walderhaug MO, Post RL, Saccomani G, Leonard RT, Briskin DP. Structural relatedness of three ion-transport adenosine triphosphatases around their active sites of phosphorylation. J Biol Chem. 1985;260:3852–3859. - PubMed
-
- Bamberg K, Sachs G. Topological analysis of H+, K+-ATPase using in vitro translation. J Biol Chem. 1994;269:16909–16919. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources


