Proton sensors in the pore domain of the cardiac voltage-gated sodium channel
- PMID: 23283979
- PMCID: PMC3576083
- DOI: 10.1074/jbc.M112.434266
Proton sensors in the pore domain of the cardiac voltage-gated sodium channel
Abstract
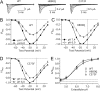
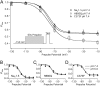
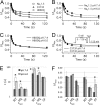
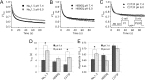
Protons impart isoform-specific modulation of inactivation in neuronal, skeletal muscle, and cardiac voltage-gated sodium (Na(V)) channels. Although the structural basis of proton block in Na(V) channels has been well described, the amino acid residues responsible for the changes in Na(V) kinetics during extracellular acidosis are as yet unknown. We expressed wild-type (WT) and two pore mutant constructs (H880Q and C373F) of the human cardiac Na(V) channel, Na(V)1.5, in Xenopus oocytes. C373F and H880Q both attenuated proton block, abolished proton modulation of use-dependent inactivation, and altered pH modulation of the steady-state and kinetic parameters of slow inactivation. Additionally, C373F significantly reduced the maximum probability of use-dependent inactivation and slow inactivation, relative to WT. H880Q also significantly reduced the maximum probability of slow inactivation and shifted the voltage dependence of activation and fast inactivation to more positive potentials, relative to WT. These data suggest that Cys-373 and His-880 in Na(V)1.5 are proton sensors for use-dependent and slow inactivation and have implications in isoform-specific modulation of Na(V) channels.
Figures



Similar articles
-
Extracellular proton modulation of the cardiac voltage-gated sodium channel, Nav1.5.Biophys J. 2011 Nov 2;101(9):2147-56. doi: 10.1016/j.bpj.2011.08.056. Epub 2011 Nov 1. Biophys J. 2011. PMID: 22067152 Free PMC article.
-
Sodium channel carboxyl-terminal residue regulates fast inactivation.J Biol Chem. 2010 Mar 19;285(12):9077-89. doi: 10.1074/jbc.M109.054940. Epub 2010 Jan 20. J Biol Chem. 2010. PMID: 20089854 Free PMC article.
-
Proton modulation of cardiac I Na: a potential arrhythmogenic trigger.Handb Exp Pharmacol. 2014;221:169-81. doi: 10.1007/978-3-642-41588-3_8. Handb Exp Pharmacol. 2014. PMID: 24737236 Review.
-
External pore residue mediates slow inactivation in mu 1 rat skeletal muscle sodium channels.J Physiol. 1996 Jul 15;494 ( Pt 2)(Pt 2):431-42. doi: 10.1113/jphysiol.1996.sp021503. J Physiol. 1996. PMID: 8842002 Free PMC article.
-
pH Modulation of Voltage-Gated Sodium Channels.Handb Exp Pharmacol. 2018;246:147-160. doi: 10.1007/164_2018_99. Handb Exp Pharmacol. 2018. PMID: 29460150 Review.
Cited by
-
Impact of Impaired Kidney Function on Arrhythmia-Promoting Cardiac Ion Channel Regulation.Int J Mol Sci. 2023 Sep 17;24(18):14198. doi: 10.3390/ijms241814198. Int J Mol Sci. 2023. PMID: 37762501 Free PMC article. Review.
-
A new polymodal gating model of the proton-activated chloride channel.PLoS Biol. 2023 Sep 15;21(9):e3002309. doi: 10.1371/journal.pbio.3002309. eCollection 2023 Sep. PLoS Biol. 2023. PMID: 37713449 Free PMC article.
-
Investigation of CACNA1I Cav3.3 Dysfunction in Hemiplegic Migraine.Front Mol Neurosci. 2022 Jul 19;15:892820. doi: 10.3389/fnmol.2022.892820. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35928792 Free PMC article.
-
Late Sodium Current of the Heart: Where Do We Stand and Where Are We Going?Pharmaceuticals (Basel). 2022 Feb 15;15(2):231. doi: 10.3390/ph15020231. Pharmaceuticals (Basel). 2022. PMID: 35215342 Free PMC article. Review.
-
Genomic and Non-Genomic Regulatory Mechanisms of the Cardiac Sodium Channel in Cardiac Arrhythmias.Int J Mol Sci. 2022 Jan 26;23(3):1381. doi: 10.3390/ijms23031381. Int J Mol Sci. 2022. PMID: 35163304 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


