A synthetic peptide with the putative iron binding motif of amyloid precursor protein (APP) does not catalytically oxidize iron
- PMID: 22916096
- PMCID: PMC3419245
- DOI: 10.1371/journal.pone.0040287
A synthetic peptide with the putative iron binding motif of amyloid precursor protein (APP) does not catalytically oxidize iron
Abstract
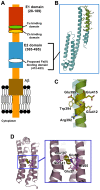
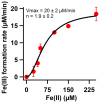
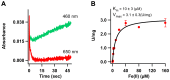
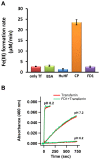
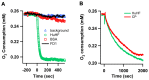
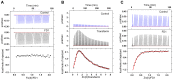
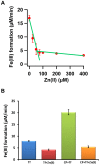
The β-amyloid precursor protein (APP), which is a key player in Alzheimer's disease, was recently reported to possess an Fe(II) binding site within its E2 domain which exhibits ferroxidase activity [Duce et al. 2010, Cell 142: 857]. The putative ligands of this site were compared to those in the ferroxidase site of ferritin. The activity was indirectly measured using transferrin, which scavenges the Fe(III) product of the reaction. A 22-residue synthetic peptide, named FD1, with the putative ferroxidase site of APP, and the E2 domain of APP were each reported to exhibit 40% of the ferroxidase activity of APP and of ceruloplasmin. It was also claimed that the ferroxidase activity of APP is inhibited by Zn(II) just as in ferritin. We measured the ferroxidase activity indirectly (i) by the incorporation of the Fe(III) product of the ferroxidase reaction into transferrin and directly (ii) by monitoring consumption of the substrate molecular oxygen. The results with the FD1 peptide were compared to the established ferroxidase activities of human H-chain ferritin and of ceruloplasmin. For FD1 we observed no activity above the background of non-enzymatic Fe(II) oxidation by molecular oxygen. Zn(II) binds to transferrin and diminishes its Fe(III) incorporation capacity and rate but it does not specifically bind to a putative ferroxidase site of FD1. Based on these results, and on comparison of the putative ligands of the ferroxidase site of APP with those of ferritin, we conclude that the previously reported results for ferroxidase activity of FD1 and - by implication - of APP should be re-evaluated.
Conflict of interest statement
Figures
Similar articles
-
The amyloid precursor protein (APP) does not have a ferroxidase site in its E2 domain.PLoS One. 2013 Aug 19;8(8):e72177. doi: 10.1371/journal.pone.0072177. eCollection 2013. PLoS One. 2013. PMID: 23977245 Free PMC article.
-
Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer's disease.Cell. 2010 Sep 17;142(6):857-67. doi: 10.1016/j.cell.2010.08.014. Cell. 2010. PMID: 20817278 Free PMC article.
-
Self-assembly is prerequisite for catalysis of Fe(II) oxidation by catalytically active subunits of ferritin.J Biol Chem. 2015 Oct 30;290(44):26801-10. doi: 10.1074/jbc.M115.678375. Epub 2015 Sep 14. J Biol Chem. 2015. PMID: 26370076 Free PMC article.
-
Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease.Biochem Soc Trans. 2008 Dec;36(Pt 6):1282-7. doi: 10.1042/BST0361282. Biochem Soc Trans. 2008. PMID: 19021541 Free PMC article. Review.
-
Identification and regulation of the high affinity binding site of the Alzheimer's disease amyloid protein precursor (APP) to glycosaminoglycans.Biochimie. 1994;76(3-4):304-11. doi: 10.1016/0300-9084(94)90163-5. Biochimie. 1994. PMID: 7819340 Review.
Cited by
-
Quantum Magnetism of the Iron Core in Ferritin Proteins-A Re-Evaluation of the Giant-Spin Model.Molecules. 2024 May 11;29(10):2254. doi: 10.3390/molecules29102254. Molecules. 2024. PMID: 38792115 Free PMC article.
-
The Case for Abandoning Therapeutic Chelation of Copper Ions in Alzheimer's Disease.Front Neurosci. 2017 Jun 2;11:317. doi: 10.3389/fnins.2017.00317. eCollection 2017. Front Neurosci. 2017. PMID: 28626387 Free PMC article. Review.
-
β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin.PLoS One. 2014 Dec 2;9(12):e114174. doi: 10.1371/journal.pone.0114174. eCollection 2014. PLoS One. 2014. PMID: 25464026 Free PMC article.
-
Iron transport across the blood-brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy.Cell Mol Life Sci. 2015 Feb;72(4):709-27. doi: 10.1007/s00018-014-1771-4. Epub 2014 Oct 30. Cell Mol Life Sci. 2015. PMID: 25355056 Free PMC article. Review.
-
The role of iron in brain ageing and neurodegenerative disorders.Lancet Neurol. 2014 Oct;13(10):1045-60. doi: 10.1016/S1474-4422(14)70117-6. Lancet Neurol. 2014. PMID: 25231526 Free PMC article. Review.
References
-
- Duce JA, Bush AI (2010) Biological metals and Alzheimer's disease: Implications for therapeutics and diagnostics. Prog Neurobiol 92: 1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical


