Regulation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel activity by cCMP
- PMID: 22715094
- PMCID: PMC3410992
- DOI: 10.1074/jbc.M112.357129
Regulation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel activity by cCMP
Abstract
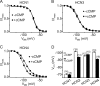
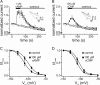
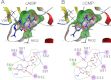
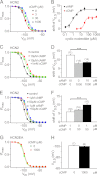
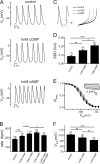
Activation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels is facilitated in vivo by direct binding of the second messenger cAMP. This process plays a fundamental role in the fine-tuning of HCN channel activity and is critical for the modulation of cardiac and neuronal rhythmicity. Here, we identify the pyrimidine cyclic nucleotide cCMP as another regulator of HCN channels. We demonstrate that cCMP shifts the activation curves of two members of the HCN channel family, HCN2 and HCN4, to more depolarized voltages. Moreover, cCMP speeds up activation and slows down deactivation kinetics of these channels. The two other members of the HCN channel family, HCN1 and HCN3, are not sensitive to cCMP. The modulatory effect of cCMP is reversible and requires the presence of a functional cyclic nucleotide-binding domain. We determined an EC(50) value of ∼30 μm for cCMP compared with 1 μm for cAMP. Notably, cCMP is a partial agonist of HCN channels, displaying an efficacy of ∼0.6. cCMP increases the frequency of pacemaker potentials from isolated sinoatrial pacemaker cells in the presence of endogenous cAMP concentrations. Electrophysiological recordings indicated that this increase is caused by a depolarizing shift in the activation curve of the native HCN current, which in turn leads to an enhancement of the slope of the diastolic depolarization of sinoatrial node cells. In conclusion, our findings establish cCMP as a gating regulator of HCN channels and indicate that this cyclic nucleotide has to be considered in HCN channel-regulated processes.
Figures

Similar articles
-
Regulation of hyperpolarization-activated HCN channel gating and cAMP modulation due to interactions of COOH terminus and core transmembrane regions.J Gen Physiol. 2001 Sep;118(3):237-50. doi: 10.1085/jgp.118.3.237. J Gen Physiol. 2001. PMID: 11524455 Free PMC article.
-
A mechanism for the auto-inhibition of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel opening and its relief by cAMP.J Biol Chem. 2014 Aug 8;289(32):22205-20. doi: 10.1074/jbc.M114.572164. Epub 2014 May 30. J Biol Chem. 2014. PMID: 24878962 Free PMC article.
-
Single-channel properties support a potential contribution of hyperpolarization-activated cyclic nucleotide-gated channels and If to cardiac arrhythmias.Circulation. 2005 Feb 1;111(4):399-404. doi: 10.1161/01.CIR.0000153799.65783.3A. Circulation. 2005. PMID: 15687126
-
Regulation of HCN Ion Channels by Non-canonical Cyclic Nucleotides.Handb Exp Pharmacol. 2017;238:123-133. doi: 10.1007/164_2016_5006. Handb Exp Pharmacol. 2017. PMID: 28181007 Review.
-
The structure of the apo cAMP-binding domain of HCN4 - a stepping stone toward understanding the cAMP-dependent modulation of the hyperpolarization-activated cyclic-nucleotide-gated ion channels.FEBS J. 2018 Jun;285(12):2182-2192. doi: 10.1111/febs.14408. Epub 2018 Mar 14. FEBS J. 2018. PMID: 29444387 Review.
Cited by
-
LRMP inhibits cAMP potentiation of HCN4 channels by disrupting intramolecular signal transduction.Elife. 2024 Apr 23;12:RP92411. doi: 10.7554/eLife.92411. Elife. 2024. PMID: 38652113 Free PMC article.
-
LRMP inhibits cAMP potentiation of HCN4 channels by disrupting intramolecular signal transduction.bioRxiv [Preprint]. 2024 Jan 24:2023.08.29.555242. doi: 10.1101/2023.08.29.555242. bioRxiv. 2024. Update in: Elife. 2024 Apr 23;12:RP92411. doi: 10.7554/eLife.92411. PMID: 37693562 Free PMC article. Updated. Preprint.
-
Modulating cyclic nucleotides pathways by bioactive compounds in combatting anxiety and depression disorders.Mol Biol Rep. 2023 Sep;50(9):7797-7814. doi: 10.1007/s11033-023-08650-8. Epub 2023 Jul 24. Mol Biol Rep. 2023. PMID: 37486442 Free PMC article. Review.
-
Photoreceptor Ion Channels in Signaling and Disease.Adv Exp Med Biol. 2023;1415:269-276. doi: 10.1007/978-3-031-27681-1_39. Adv Exp Med Biol. 2023. PMID: 37440044
-
Putative Nucleotide-Based Second Messengers in the Archaeal Model Organisms Haloferax volcanii and Sulfolobus acidocaldarius.Front Microbiol. 2021 Nov 22;12:779012. doi: 10.3389/fmicb.2021.779012. eCollection 2021. Front Microbiol. 2021. PMID: 34880846 Free PMC article.
References
-
- Biel M., Wahl-Schott C., Michalakis S., Zong X. (2009) Hyperpolarization-activated cation channels: from genes to function. Physiol. Rev. 89, 847–885 - PubMed
-
- DiFrancesco D. (2010) The role of the funny current in pacemaker activity. Circ. Res. 106, 434–446 - PubMed
-
- Frère S. G., Kuisle M., Lüthi A. (2004) Regulation of recombinant and native hyperpolarization-activated cation channels. Mol. Neurobiol. 30, 279–305 - PubMed
-
- Ludwig A., Zong X., Jeglitsch M., Hofmann F., Biel M. (1998) A family of hyperpolarization-activated mammalian cation channels. Nature 393, 587–591 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases


