ELIGLUSTAT TARTRATE: Glucosylceramide Synthase Inhibitor Treatment of Type 1 Gaucher Disease
- PMID: 22563139
- PMCID: PMC3340614
ELIGLUSTAT TARTRATE: Glucosylceramide Synthase Inhibitor Treatment of Type 1 Gaucher Disease
Abstract
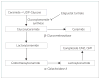
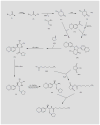
Eliglustat tartrate (Genz-112638) is a novel, orally administered agent currently in development for the treatment of lysosomal storage disorders, including type 1 Gaucher disease and Fabry disease. This glucosylceramide analogue acts as an inhibitor of glucosylceramide synthase, a Golgi complex enzyme that catalyzes the formation of glucosylceramide from ceramide and UDP-glucose and is the first step in the formation of glucocerebroside-based glycosphingolipids. Pre-clinical pharmacological studies demonstrate that the agent has a high therapeutic index, excellent oral bioavailability and limited toxicity. Phase I studies in healthy volunteers revealed limited toxicity with an excellent pharmacodynamic response, as measured by decreased plasma glucosylceramide concentrations. Phase II studies in patients with type 1 Gaucher disease have demonstrated promising clinical responses, as measured by decreases in spleen size, improvement in hemoglobin concentrations and increased platelet counts. Two randomized phase III trials testing the efficacy and safety of eliglustat tartrate are currently in progress.
Figures
Similar articles
-
Eliglustat tartrate, an orally active glucocerebroside synthase inhibitor for the potential treatment of Gaucher disease and other lysosomal storage diseases.Curr Opin Investig Drugs. 2010 Oct;11(10):1169-81. Curr Opin Investig Drugs. 2010. PMID: 20872320 Review.
-
Eliglustat tartrate, a prototypic glucosylceramide synthase inhibitor.Expert Rev Endocrinol Metab. 2013 Nov;8(6):491-504. doi: 10.1586/17446651.2013.846213. Expert Rev Endocrinol Metab. 2013. PMID: 30736134
-
Inhibitors of Glucosylceramide Synthase.Methods Mol Biol. 2023;2613:271-288. doi: 10.1007/978-1-0716-2910-9_20. Methods Mol Biol. 2023. PMID: 36587085
-
A phase 2 study of eliglustat tartrate (Genz-112638), an oral substrate reduction therapy for Gaucher disease type 1.Blood. 2010 Aug 12;116(6):893-9. doi: 10.1182/blood-2010-03-273151. Epub 2010 May 3. Blood. 2010. PMID: 20439622 Free PMC article. Clinical Trial.
-
Profile of eliglustat tartrate in the management of Gaucher disease.Ther Clin Risk Manag. 2016 Jan 11;12:53-8. doi: 10.2147/TCRM.S73226. eCollection 2016. Ther Clin Risk Manag. 2016. PMID: 26811686 Free PMC article. Review.
Cited by
-
Targeting sphingolipid metabolism in chronic lymphocytic leukemia.Clin Exp Med. 2024 Jul 30;24(1):174. doi: 10.1007/s10238-024-01440-x. Clin Exp Med. 2024. PMID: 39078421 Free PMC article.
-
A Novel Cytotoxic Mechanism for Triple-Negative Breast Cancer Cells Induced by the Type II Heat-Labile Enterotoxin LT-IIc through Ganglioside Ligation.Toxins (Basel). 2024 Jul 11;16(7):311. doi: 10.3390/toxins16070311. Toxins (Basel). 2024. PMID: 39057951 Free PMC article.
-
Bioinformatics to Identify Biomarkers of Diabetic Nephropathy based on Sphingolipid Metabolism and their Molecular Mechanisms.Curr Diabetes Rev. 2024;21(2):e070524229720. doi: 10.2174/0115733998297749240418071555. Curr Diabetes Rev. 2024. PMID: 38712372
-
Drug Repurposing and Lysosomal Storage Disorders: A Trick to Treat.Genes (Basel). 2024 Feb 25;15(3):290. doi: 10.3390/genes15030290. Genes (Basel). 2024. PMID: 38540351 Free PMC article. Review.
-
GBA Regulates EMT/MET and Chemoresistance in Squamous Cell Carcinoma Cells by Modulating the Cellular Glycosphingolipid Profile.Cells. 2023 Jul 18;12(14):1886. doi: 10.3390/cells12141886. Cells. 2023. PMID: 37508550 Free PMC article.
References
-
- Dellaria JF, Jr, Santarsiero BD. Enantioselective synthesis of alpha-amino acid derivatives via the stereoselective alkylation of a homochiral glycine enolate synthon. J Org Chem. 1989;54(16):3916–26.
-
- Siegel C, Hirth BH Genzyme Corp. CA 2453978, EP 1409467, EP 2067775, JP 2005255686, JP 2005502635, JP 2010095546, US 2003050299, US 6855830, WO 2003008399 Synthesis of UDP-glucose:N-acyl-sphingosine glucosyltransferase inhibitors.
-
- Charrow J, Andersson HC, Kaplan P, et al. The Gaucher registry: Demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Intern Med. 2000;160(18):2835–43. - PubMed
-
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249–54. - PubMed
-
- Grabowski GA. Gaucher disease: Gene frequencies and genotype/phenotype correlations. Genet Test. 1997;1(1):5–12. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

