Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia
- PMID: 22440736
- PMCID: PMC3319975
- DOI: 10.1126/scitranslmed.3003122
Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia
Abstract
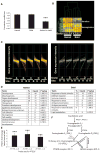
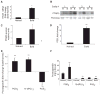
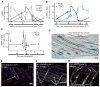
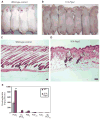
Testosterone is necessary for the development of male pattern baldness, known as androgenetic alopecia (AGA); yet, the mechanisms for decreased hair growth in this disorder are unclear. We show that prostaglandin D(2) synthase (PTGDS) is elevated at the mRNA and protein levels in bald scalp compared to haired scalp of men with AGA. The product of PTGDS enzyme activity, prostaglandin D(2) (PGD(2)), is similarly elevated in bald scalp. During normal follicle cycling in mice, Ptgds and PGD(2) levels increase immediately preceding the regression phase, suggesting an inhibitory effect on hair growth. We show that PGD(2) inhibits hair growth in explanted human hair follicles and when applied topically to mice. Hair growth inhibition requires the PGD(2) receptor G protein (heterotrimeric guanine nucleotide)-coupled receptor 44 (GPR44), but not the PGD(2) receptor 1 (PTGDR). Furthermore, we find that a transgenic mouse, K14-Ptgs2, which targets prostaglandin-endoperoxide synthase 2 expression to the skin, demonstrates elevated levels of PGD(2) in the skin and develops alopecia, follicular miniaturization, and sebaceous gland hyperplasia, which are all hallmarks of human AGA. These results define PGD(2) as an inhibitor of hair growth in AGA and suggest the PGD(2)-GPR44 pathway as a potential target for treatment.
Conflict of interest statement
Figures

Similar articles
-
Does prostaglandin D2 hold the cure to male pattern baldness?Exp Dermatol. 2014 Apr;23(4):224-7. doi: 10.1111/exd.12348. Exp Dermatol. 2014. PMID: 24521203 Free PMC article. Review.
-
15-deoxy prostaglandin J2, the nonenzymatic metabolite of prostaglandin D2, induces apoptosis in keratinocytes of human hair follicles: a possible explanation for prostaglandin D2-mediated inhibition of hair growth.Naunyn Schmiedebergs Arch Pharmacol. 2016 Jul;389(7):809-13. doi: 10.1007/s00210-016-1257-z. Epub 2016 May 17. Naunyn Schmiedebergs Arch Pharmacol. 2016. PMID: 27185495
-
Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44.J Invest Dermatol. 2013 Apr;133(4):881-9. doi: 10.1038/jid.2012.398. Epub 2012 Nov 29. J Invest Dermatol. 2013. PMID: 23190891 Free PMC article.
-
Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells.J Clin Invest. 2011 Feb;121(2):613-22. doi: 10.1172/JCI44478. Epub 2011 Jan 4. J Clin Invest. 2011. PMID: 21206086 Free PMC article.
-
Multi-therapies in androgenetic alopecia: review and clinical experiences.Dermatol Ther. 2016 Nov;29(6):424-432. doi: 10.1111/dth.12390. Epub 2016 Jul 18. Dermatol Ther. 2016. PMID: 27424565 Review.
Cited by
-
Disturbance of Immune Microenvironment in Androgenetic Alopecia through Spatial Transcriptomics.Int J Mol Sci. 2024 Aug 20;25(16):9031. doi: 10.3390/ijms25169031. Int J Mol Sci. 2024. PMID: 39201715 Free PMC article.
-
Network Pharmacology Reveals Curcuma aeruginosa Roxb. Regulates MAPK and HIF-1 Pathways to Treat Androgenetic Alopecia.Biology (Basel). 2024 Jul 4;13(7):497. doi: 10.3390/biology13070497. Biology (Basel). 2024. PMID: 39056691 Free PMC article.
-
Metabolomics reveals metabolites associated with hair follicle cycle in cashmere goats.BMC Vet Res. 2024 May 17;20(1):208. doi: 10.1186/s12917-024-04057-0. BMC Vet Res. 2024. PMID: 38760765 Free PMC article.
-
The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia.Int J Mol Sci. 2024 Feb 22;25(5):2542. doi: 10.3390/ijms25052542. Int J Mol Sci. 2024. PMID: 38473791 Free PMC article. Review.
-
Signaling pathways in hair aging.Front Cell Dev Biol. 2023 Nov 16;11:1278278. doi: 10.3389/fcell.2023.1278278. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38033857 Free PMC article. Review.
References
-
- Alsantali A, Shapiro J. Androgens and hair loss. Curr Opin Endocrinol Diabetes Obes. 2009;16:246–253. - PubMed
-
- Richards JB, Yuan X, Geller F, Waterworth D, Bataille V, Glass D, Song K, Waeber G, Vollenweider P, Aben KK, Kiemeney LA, Walters B, Soranzo N, Thorsteinsdottir U, Kong A, Rafnar T, Deloukas P, Sulem P, Stefansson H, Stefansson K, Spector TD, Mooser V. Male-pattern baldness susceptibility locus at 20p11. Nat Genet. 2008;40:1282–1284. - PMC - PubMed
-
- Hillmer AM, Hanneken S, Ritzmann S, Becker T, Freudenberg J, Brockschmidt FF, Flaquer A, Freudenberg-Hua Y, Jamra RA, Metzen C, Heyn U, Schweiger N, Betz RC, Blaumeiser B, Hampe J, Schreiber S, Schulze TG, Hennies HC, Schumacher J, Propping P, Ruzicka T, Cichon S, Wienker TF, Kruse R, Nothen MM. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am J Hum Genet. 2005;77:140–148. - PMC - PubMed
-
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. - PubMed
-
- Oh BR, Kim SJ, Moon JD, Kim HN, Kwon DD, Won YH, Ryu SB, Park YI. Association of benign prostatic hyperplasia with male pattern baldness. Urology. 1998;51:744–748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 AR055666-05/AR/NIAMS NIH HHS/United States
- K08 AR055666/AR/NIAMS NIH HHS/United States
- K08AR055666/AR/NIAMS NIH HHS/United States
- K08 AR055666-03/AR/NIAMS NIH HHS/United States
- R01 AR055309/AR/NIAMS NIH HHS/United States
- R01 AR064297/AR/NIAMS NIH HHS/United States
- P30-AR057217/AR/NIAMS NIH HHS/United States
- R01-AR46837/AR/NIAMS NIH HHS/United States
- K08 AR055666-01A1/AR/NIAMS NIH HHS/United States
- K08 AR055666-04/AR/NIAMS NIH HHS/United States
- K08 AR055666-06/AR/NIAMS NIH HHS/United States
- K08 AR055666-02/AR/NIAMS NIH HHS/United States
- R01 AR046837/AR/NIAMS NIH HHS/United States
- R01-AR055309/AR/NIAMS NIH HHS/United States
- P30 AR057217/AR/NIAMS NIH HHS/United States
- 5-P30-AR-057217-02/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous


