Effects of lesions of the amygdala central nucleus on autoshaped lever pressing
- PMID: 22386516
- PMCID: PMC3319525
- DOI: 10.1016/j.brainres.2012.02.029
Effects of lesions of the amygdala central nucleus on autoshaped lever pressing
Abstract
Neutral cues paired with rewards often appear to acquire motivational significance, as if the incentive motivational value of the reward is transferred to the cue. Such cues have been reported to modulate the performance of instrumental action (Pavlovian-instrumental transfer, PIT), serve as conditioned reinforcers in the establishment of new learning, and be the targets of approach and other cue-directed behaviors. Here we examined the effects of lesions of the amygdala central nucleus (CeA) on the acquisition of discriminative autoshaped lever-pressing. Insertion of one lever into the experimental chamber was reinforced by sucrose delivery, but insertion of another lever was not reinforced. Although sucrose delivery was not contingent on lever pressing, both CeA- and sham-lesioned rats rapidly came to press the reinforced but not the nonreinforced lever. Despite their showing little evidence of impairments in autoshaped lever pressing, these same CeA-lesioned rats showed significant deficits in the expression of PIT in a subsequent phase of the experiment. The lack of impaired autoshaping in CeA-lesioned rats contrasts with effects previously reported for conditioned orienting responses (ORs) and for other putative measures of incentive learning including PIT and conditioned approach to visual cues.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

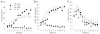
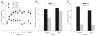
Similar articles
-
Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing.Neurobiol Learn Mem. 2012 May;97(4):441-51. doi: 10.1016/j.nlm.2012.03.008. Epub 2012 Mar 26. Neurobiol Learn Mem. 2012. PMID: 22469749 Free PMC article.
-
Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats.J Neurosci. 2001 Nov 15;21(22):9018-26. doi: 10.1523/JNEUROSCI.21-22-09018.2001. J Neurosci. 2001. PMID: 11698612 Free PMC article.
-
Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer.J Neurosci. 2005 Jan 26;25(4):962-70. doi: 10.1523/JNEUROSCI.4507-04.2005. J Neurosci. 2005. PMID: 15673677 Free PMC article.
-
Role of amygdala central nucleus in the potentiation of consuming and instrumental lever-pressing for sucrose by cues for the presentation or interruption of sucrose delivery in rats.Behav Neurosci. 2014 Feb;128(1):71-82. doi: 10.1037/a0035445. Behav Neurosci. 2014. PMID: 24512067 Free PMC article.
-
Unraveling the influence of Pavlovian cues on decision-making: A pre-registered meta-analysis on Pavlovian-to-instrumental transfer.Neurosci Biobehav Rev. 2024 Sep;164:105829. doi: 10.1016/j.neubiorev.2024.105829. Epub 2024 Jul 27. Neurosci Biobehav Rev. 2024. PMID: 39074674 Review.
Cited by
-
Incentive motivation: 'wanting' roles of central amygdala circuitry.Behav Brain Res. 2021 Aug 6;411:113376. doi: 10.1016/j.bbr.2021.113376. Epub 2021 May 20. Behav Brain Res. 2021. PMID: 34023307 Free PMC article. Review.
-
Sign-tracking behavior is sensitive to outcome devaluation in a devaluation context-dependent manner: implications for analyzing habitual behavior.Learn Mem. 2020 Mar 16;27(4):136-149. doi: 10.1101/lm.051144.119. Print 2020 Apr. Learn Mem. 2020. PMID: 32179656 Free PMC article.
-
Mapping sign-tracking and goal-tracking onto human behaviors.Neurosci Biobehav Rev. 2020 Apr;111:84-94. doi: 10.1016/j.neubiorev.2020.01.018. Epub 2020 Jan 20. Neurosci Biobehav Rev. 2020. PMID: 31972203 Free PMC article. Review.
-
Neural circuits linking sleep and addiction: Animal models to understand why select individuals are more vulnerable to substance use disorders after sleep deprivation.Neurosci Biobehav Rev. 2020 Jan;108:435-444. doi: 10.1016/j.neubiorev.2019.11.007. Epub 2019 Nov 19. Neurosci Biobehav Rev. 2020. PMID: 31756346 Free PMC article. Review.
-
Voluntary alcohol access during adolescence/early adulthood, but not during adulthood, causes faster omission contingency learning.Behav Brain Res. 2019 Sep 16;370:111918. doi: 10.1016/j.bbr.2019.111918. Epub 2019 May 13. Behav Brain Res. 2019. PMID: 31095991 Free PMC article.
References
-
- Berridge KC. Measuring hedonic impact in animals and infants: Microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. - PubMed
-
- Berridge KC. Reward learning: Reinforcement, incentives, and expectations. Psychol Learn Motiv Advances Res Theory. 2001;40:223–278.
-
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behavior. 2004;81:179–209. - PubMed
-
- Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Hillsdale, NJ: Lawrence Eribaum Associates; 1977. pp. 67–97.
-
- Burns LH, Robbins TW, Everitt BJ. Differential-effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor-activity potentiated by intraaccumbens infusions of D-amphetamine. Behav Brain Res. 1993;55:167–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


