Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection
- PMID: 22034068
- PMCID: PMC3288456
- DOI: 10.1002/art.33444
Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection
Abstract
Objective: Mechanical injury induces cell death in cartilage and triggers a remodeling process that ultimately can manifest as osteoarthritis. Autophagy is a process for turnover of intracellular organelles and macromolecules that protects cells during stress responses. This study was undertaken to determine changes in and functions of autophagy following mechanical injury to cartilage.
Methods: Bovine and human cartilage explants were subjected to mechanical impact (40% strain for 500 msec). Cell viability, sulfated glycosaminoglycan (sGAG) release, and changes in the levels of the autophagy markers ULK1, beclin 1, and microtubule-associated protein 1 light chain 3 (LC3) were evaluated. Cartilage explants were treated with the mammalian target of rapamycin complex 1 (mTORC-1) inhibitor and the autophagy inducer rapamycin and tested for protective effects against mechanical injury. Explants were also treated with the cell death inducers nitric oxide and tumor necrosis factor α (TNFα) plus actinomycin D, and the proinflammatory cytokine interleukin-1α (IL-1α).
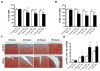
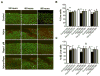
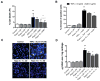
Results: Mechanical injury induced cell death and loss of sGAG in a time-dependent manner. This was associated with significantly decreased ULK1, beclin 1, and LC3 expression in the cartilage superficial zone (P < 0.05) 48 hours after injury. The levels of LC3-II were increased 24 hours after injury but decreased at 48 and 96 hours. Rapamycin enhanced expression of autophagy regulators and prevented cell death and sGAG loss in mechanically injured explants. Rapamycin also protected against cell death induced by sodium nitroprusside and TNFα plus actinomycin D and prevented sGAG loss induced by IL-1α.
Conclusion: Our findings indicate that mechanical injury leads to suppression of autophagy, predominantly in the superficial zone where most of the cell death occurs. Pharmacologic inhibition of mTORC-1, at least in part by enhancement of autophagy, prevents cell and matrix damage, suggesting a novel approach for chondroprotection.
Copyright © 2012 by the American College of Rheumatology.
Figures


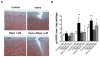
Similar articles
-
Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis.Arthritis Rheum. 2010 Mar;62(3):791-801. doi: 10.1002/art.27305. Arthritis Rheum. 2010. PMID: 20187128 Free PMC article.
-
Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis.Ann Rheum Dis. 2015 Jul;74(7):1432-40. doi: 10.1136/annrheumdis-2013-204599. Epub 2014 Mar 20. Ann Rheum Dis. 2015. PMID: 24651621
-
Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes.Arthritis Rheumatol. 2015 Apr;67(4):966-76. doi: 10.1002/art.39025. Arthritis Rheumatol. 2015. PMID: 25605458 Free PMC article.
-
Autophagy in osteoarthritis.Joint Bone Spine. 2016 Mar;83(2):143-8. doi: 10.1016/j.jbspin.2015.06.009. Epub 2015 Oct 6. Joint Bone Spine. 2016. PMID: 26453105 Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
New insights into the mechanisms and therapeutic strategies of chondrocyte autophagy in osteoarthritis.J Mol Med (Berl). 2024 Oct;102(10):1229-1244. doi: 10.1007/s00109-024-02473-1. Epub 2024 Aug 15. J Mol Med (Berl). 2024. PMID: 39145815 Review.
-
Autophagy in Osteoarthritis: A Double-Edged Sword in Cartilage Aging and Mechanical Stress Response: A Systematic Review.J Clin Med. 2024 May 20;13(10):3005. doi: 10.3390/jcm13103005. J Clin Med. 2024. PMID: 38792546 Free PMC article. Review.
-
A loss of primary cilia by a reduction in mTOR signaling correlates with age-related deteriorations in condylar cartilage.Geroscience. 2024 Dec;46(6):5995-6007. doi: 10.1007/s11357-024-01143-x. Epub 2024 Mar 25. Geroscience. 2024. PMID: 38526843 Free PMC article.
-
Role of Necroptosis in Intervertebral Disc Degeneration.Int J Mol Sci. 2023 Oct 18;24(20):15292. doi: 10.3390/ijms242015292. Int J Mol Sci. 2023. PMID: 37894970 Free PMC article. Review.
-
Osteoarthritis: Role of Peroxisome Proliferator-Activated Receptors.Int J Mol Sci. 2023 Aug 24;24(17):13137. doi: 10.3390/ijms241713137. Int J Mol Sci. 2023. PMID: 37685944 Free PMC article. Review.
References
-
- Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–93. - PubMed
-
- Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48:1292–301. - PubMed
-
- Sui Y, Lee JH, DiMicco MA, Vanderploeg EJ, Blake SM, Hung HH, et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009;60:2985–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials


