Neural coding of continuous speech in auditory cortex during monaural and dichotic listening
- PMID: 21975452
- PMCID: PMC3570829
- DOI: 10.1152/jn.00297.2011
Neural coding of continuous speech in auditory cortex during monaural and dichotic listening
Abstract
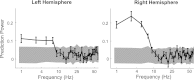
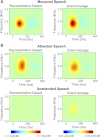
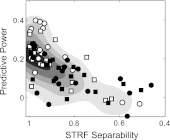
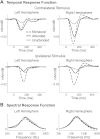
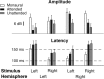
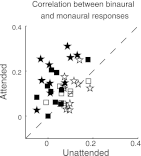
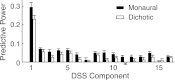
The cortical representation of the acoustic features of continuous speech is the foundation of speech perception. In this study, noninvasive magnetoencephalography (MEG) recordings are obtained from human subjects actively listening to spoken narratives, in both simple and cocktail party-like auditory scenes. By modeling how acoustic features of speech are encoded in ongoing MEG activity as a spectrotemporal response function, we demonstrate that the slow temporal modulations of speech in a broad spectral region are represented bilaterally in auditory cortex by a phase-locked temporal code. For speech presented monaurally to either ear, this phase-locked response is always more faithful in the right hemisphere, but with a shorter latency in the hemisphere contralateral to the stimulated ear. When different spoken narratives are presented to each ear simultaneously (dichotic listening), the resulting cortical neural activity precisely encodes the acoustic features of both of the spoken narratives, but slightly weakened and delayed compared with the monaural response. Critically, the early sensory response to the attended speech is considerably stronger than that to the unattended speech, demonstrating top-down attentional gain control. This attentional gain is substantial even during the subjects' very first exposure to the speech mixture and therefore largely independent of knowledge of the speech content. Together, these findings characterize how the spectrotemporal features of speech are encoded in human auditory cortex and establish a single-trial-based paradigm to study the neural basis underlying the cocktail party phenomenon.
Figures


Similar articles
-
Cortical Representations of Speech in a Multitalker Auditory Scene.J Neurosci. 2017 Sep 20;37(38):9189-9196. doi: 10.1523/JNEUROSCI.0938-17.2017. Epub 2017 Aug 18. J Neurosci. 2017. PMID: 28821680 Free PMC article.
-
Left Superior Temporal Gyrus Is Coupled to Attended Speech in a Cocktail-Party Auditory Scene.J Neurosci. 2016 Feb 3;36(5):1596-606. doi: 10.1523/JNEUROSCI.1730-15.2016. J Neurosci. 2016. PMID: 26843641 Free PMC article.
-
Robust cortical encoding of slow temporal modulations of speech.Adv Exp Med Biol. 2013;787:373-81. doi: 10.1007/978-1-4614-1590-9_41. Adv Exp Med Biol. 2013. PMID: 23716243
-
The encoding of auditory objects in auditory cortex: insights from magnetoencephalography.Int J Psychophysiol. 2015 Feb;95(2):184-90. doi: 10.1016/j.ijpsycho.2014.05.005. Epub 2014 May 16. Int J Psychophysiol. 2015. PMID: 24841996 Free PMC article. Review.
-
Neural Encoding of Attended Continuous Speech under Different Types of Interference.J Cogn Neurosci. 2018 Nov;30(11):1606-1619. doi: 10.1162/jocn_a_01303. Epub 2018 Jul 13. J Cogn Neurosci. 2018. PMID: 30004849 Review.
Cited by
-
Distinct roles of SNR, speech Intelligibility, and attentional effort on neural speech tracking in noise.bioRxiv [Preprint]. 2024 Oct 12:2024.10.10.616515. doi: 10.1101/2024.10.10.616515. bioRxiv. 2024. PMID: 39416110 Free PMC article. Preprint.
-
ADT Network: A Novel Nonlinear Method for Decoding Speech Envelopes From EEG Signals.Trends Hear. 2024 Jan-Dec;28:23312165241282872. doi: 10.1177/23312165241282872. Trends Hear. 2024. PMID: 39397786 Free PMC article.
-
FMRI speech tracking in primary and non-primary auditory cortex while listening to noisy scenes.Commun Biol. 2024 Sep 30;7(1):1217. doi: 10.1038/s42003-024-06913-z. Commun Biol. 2024. PMID: 39349723 Free PMC article.
-
Cortical mechanisms of across-ear speech integration investigated using functional near-infrared spectroscopy (fNIRS).PLoS One. 2024 Sep 18;19(9):e0307158. doi: 10.1371/journal.pone.0307158. eCollection 2024. PLoS One. 2024. PMID: 39292701 Free PMC article.
-
Neurophysiology of Effortful Listening: Decoupling Motivational Modulation from Task Demands.J Neurosci. 2024 Oct 30;44(44):e0589242024. doi: 10.1523/JNEUROSCI.0589-24.2024. J Neurosci. 2024. PMID: 39261007
References
-
- Aiken SJ, Picton TW. Human cortical responses to the speech envelope. Ear Hear 29: 139–157, 2008 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


