N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis
- PMID: 21965673
- PMCID: PMC3220534
- DOI: 10.1074/jbc.M111.277814
N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis
Abstract
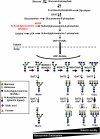
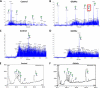
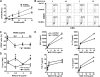
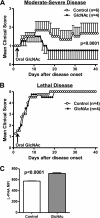
Current treatments and emerging oral therapies for multiple sclerosis (MS) are limited by effectiveness, cost, and/or toxicity. Genetic and environmental factors that alter the branching of Asn (N)-linked glycans result in T cell hyperactivity, promote spontaneous inflammatory demyelination and neurodegeneration in mice, and converge to regulate the risk of MS. The sugar N-acetylglucosamine (GlcNAc) enhances N-glycan branching and inhibits T cell activity and adoptive transfer experimental autoimmune encephalomyelitis (EAE). Here, we report that oral GlcNAc inhibits T-helper 1 (Th1) and T-helper 17 (Th17) responses and attenuates the clinical severity of myelin oligodendrocyte glycoprotein (MOG)-induced EAE when administered after disease onset. Oral GlcNAc increased expression of branched N-glycans in T cells in vivo as shown by high pH anion exchange chromatography, MALDI-TOF mass spectroscopy and FACS analysis with the plant lectin l-phytohemagglutinin. Initiating oral GlcNAc treatment on the second day of clinical disease inhibited MOG-induced EAE as well as secretion of interferon-γ, tumor necrosis factor-α, interleukin-17, and interleukin-22. In the more severe 2D2 T cell receptor transgenic EAE model, oral GlcNAc initiated after disease onset also inhibits clinical disease, except for those with rapid lethal progression. These data suggest that oral GlcNAc may provide an inexpensive and nontoxic oral therapeutic agent for MS that directly targets an underlying molecular mechanism causal of disease.
Figures

Similar articles
-
Increasing cell permeability of N-acetylglucosamine via 6-acetylation enhances capacity to suppress T-helper 1 (TH1)/TH17 responses and autoimmunity.PLoS One. 2019 Mar 26;14(3):e0214253. doi: 10.1371/journal.pone.0214253. eCollection 2019. PLoS One. 2019. PMID: 30913278 Free PMC article.
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
-
Using EAE to better understand principles of immune function and autoimmune pathology.J Autoimmun. 2013 Sep;45:31-9. doi: 10.1016/j.jaut.2013.06.008. Epub 2013 Jul 9. J Autoimmun. 2013. PMID: 23849779 Free PMC article. Review.
-
Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis.PLoS One. 2010 Nov 29;5(11):e15531. doi: 10.1371/journal.pone.0015531. PLoS One. 2010. PMID: 21209700 Free PMC article.
-
BJ-2266 ameliorates experimental autoimmune encephalomyelitis through down-regulation of the JAK/STAT signaling pathway.Eur J Immunol. 2017 Sep;47(9):1488-1500. doi: 10.1002/eji.201646860. Epub 2017 Jul 26. Eur J Immunol. 2017. PMID: 28681958
Cited by
-
The Role of miR-155 in Modulating Gene Expression in CD4+ T Cells: Insights into Alternative Immune Pathways in Autoimmune Encephalomyelitis.Int J Mol Sci. 2024 Oct 22;25(21):11355. doi: 10.3390/ijms252111355. Int J Mol Sci. 2024. PMID: 39518908 Free PMC article.
-
Synthesis and anti-inflammatory activities of two new N-acetyl glucosamine derivatives.Sci Rep. 2024 May 14;14(1):11079. doi: 10.1038/s41598-024-61780-2. Sci Rep. 2024. PMID: 38745047 Free PMC article.
-
The Immunometabolic Gene N-Acetylglucosamine Kinase Is Uniquely Involved in the Heritability of Multiple Sclerosis Severity.Int J Mol Sci. 2024 Mar 28;25(7):3803. doi: 10.3390/ijms25073803. Int J Mol Sci. 2024. PMID: 38612613 Free PMC article.
-
Effect of Chitosan Degradation Products, Glucosamine and Chitosan Oligosaccharide, on Osteoclastic Differentiation.BioTech (Basel). 2024 Mar 6;13(1):6. doi: 10.3390/biotech13010006. BioTech (Basel). 2024. PMID: 38534915 Free PMC article.
-
Salivary signatures of oral-brain communication in sleep bruxers.Front Cell Infect Microbiol. 2023 Dec 6;13:1321855. doi: 10.3389/fcimb.2023.1321855. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38125907 Free PMC article.
References
-
- Cohen J. A., Barkhof F., Comi G., Hartung H. P., Khatri B. O., Montalban X., Pelletier J., Capra R., Gallo P., Izquierdo G., Tiel-Wilck K., de Vera A., Jin J., Stites T., Wu S., Aradhye S., Kappos L. (2010) N. Engl. J. Med. 362, 402–415 - PubMed
-
- Kappos L., Radue E. W., O'Connor P., Polman C., Hohlfeld R., Calabresi P., Selmaj K., Agoropoulou C., Leyk M., Zhang-Auberson L., Burtin P. (2010) N. Engl. J. Med. 362, 387–401 - PubMed
-
- Giovannoni G., Comi G., Cook S., Rammohan K., Rieckmann P., Soelberg Sørensen P., Vermersch P., Chang P., Hamlett A., Musch B., Greenberg S. J. (2010) N. Engl. J. Med. 362, 416–426 - PubMed
-
- Baranzini S. E., Mudge J., van Velkinburgh J. C., Khankhanian P., Khrebtukova I., Miller N. A., Zhang L., Farmer A. D., Bell C. J., Kim R. W., May G. D., Woodward J. E., Caillier S. J., McElroy J. P., Gomez R., Pando M. J., Clendenen L. E., Ganusova E. E., Schilkey F. D., Ramaraj T., Khan O. A., Huntley J. J., Luo S., Kwok P. Y., Wu T. D., Schroth G. P., Oksenberg J. R., Hauser S. L., Kingsmore S. F. (2010) Nature 464, 1351–1356 - PMC - PubMed
-
- Ebers G. C., Bulman D. E., Sadovnick A. D., Paty D. W., Warren S., Hader W., Murray T. J., Seland T. P., Duquette P., Grey T., et al. (1986) N. Engl. J. Med. 315, 1638–1642 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


