Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies
- PMID: 21700325
- PMCID: PMC3132082
- DOI: 10.1016/j.cell.2011.06.001
Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies
Abstract
Parkinson's disease (PD), an adult neurodegenerative disorder, has been clinically linked to the lysosomal storage disorder Gaucher disease (GD), but the mechanistic connection is not known. Here, we show that functional loss of GD-linked glucocerebrosidase (GCase) in primary cultures or human iPS neurons compromises lysosomal protein degradation, causes accumulation of α-synuclein (α-syn), and results in neurotoxicity through aggregation-dependent mechanisms. Glucosylceramide (GlcCer), the GCase substrate, directly influenced amyloid formation of purified α-syn by stabilizing soluble oligomeric intermediates. We further demonstrate that α-syn inhibits the lysosomal activity of normal GCase in neurons and idiopathic PD brain, suggesting that GCase depletion contributes to the pathogenesis of sporadic synucleinopathies. These findings suggest that the bidirectional effect of α-syn and GCase forms a positive feedback loop that may lead to a self-propagating disease. Therefore, improved targeting of GCase to lysosomes may represent a specific therapeutic approach for PD and other synucleinopathies.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
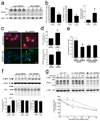
 p<0.01 compared to wt α-syn, wt and GD tau, and wt and GD Htt; *p<0.05 compared to wt tau).
p<0.01 compared to wt α-syn, wt and GD tau, and wt and GD Htt; *p<0.05 compared to wt tau).


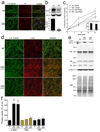
Comment in
-
A feedforward loop links Gaucher and Parkinson's diseases?Cell. 2011 Jul 8;146(1):9-11. doi: 10.1016/j.cell.2011.06.031. Cell. 2011. PMID: 21729776
-
Looping the link between Gaucher and Parkinson's disease.Clin Genet. 2011 Nov;80(5):426-7. doi: 10.1111/j.1399-0004.2011.01768.x. Clin Genet. 2011. PMID: 21883165 No abstract available.
-
Links between glucocerebrosidase and alpha-synuclein revealed.Mov Disord. 2011 Oct;26(12):2177. doi: 10.1002/mds.23985. Mov Disord. 2011. PMID: 22319791 No abstract available.
Similar articles
-
Activation of β-Glucocerebrosidase Reduces Pathological α-Synuclein and Restores Lysosomal Function in Parkinson's Patient Midbrain Neurons.J Neurosci. 2016 Jul 20;36(29):7693-706. doi: 10.1523/JNEUROSCI.0628-16.2016. J Neurosci. 2016. PMID: 27445146 Free PMC article.
-
Glucocerebrosidase deficiency promotes release of α-synuclein fibrils from cultured neurons.Hum Mol Genet. 2020 Jun 27;29(10):1716-1728. doi: 10.1093/hmg/ddaa085. Hum Mol Genet. 2020. PMID: 32391886 Free PMC article.
-
Lysosomal functions and dysfunctions: Molecular and cellular mechanisms underlying Gaucher disease and its association with Parkinson disease.Adv Drug Deliv Rev. 2022 Aug;187:114402. doi: 10.1016/j.addr.2022.114402. Epub 2022 Jun 25. Adv Drug Deliv Rev. 2022. PMID: 35764179 Review.
-
Glucocerebrosidase dysfunction in neurodegenerative disease.Essays Biochem. 2021 Dec 22;65(7):873-883. doi: 10.1042/EBC20210018. Essays Biochem. 2021. PMID: 34528667 Review.
-
Alpha-synuclein interacts with Glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases.J Biol Chem. 2011 Aug 12;286(32):28080-8. doi: 10.1074/jbc.M111.237859. Epub 2011 Jun 8. J Biol Chem. 2011. PMID: 21653695 Free PMC article.
Cited by
-
Synaptic vesicle endocytosis deficits underlie GBA-linked cognitive dysfunction in Parkinson's disease and Dementia with Lewy bodies.bioRxiv [Preprint]. 2024 Oct 23:2024.10.23.619548. doi: 10.1101/2024.10.23.619548. bioRxiv. 2024. PMID: 39484386 Free PMC article. Preprint.
-
CSF d18:1 sphingolipid species in Parkinson disease and dementia with Lewy bodies with and without GBA1 variants.NPJ Parkinsons Dis. 2024 Oct 24;10(1):198. doi: 10.1038/s41531-024-00820-0. NPJ Parkinsons Dis. 2024. PMID: 39448669 Free PMC article.
-
The role of polo-like kinases 2 in the proteasomal and lysosomal degradation of alpha-synuclein in neurons.FASEB J. 2024 Oct;38(20):e70121. doi: 10.1096/fj.202401035R. FASEB J. 2024. PMID: 39436202 Free PMC article.
-
Exploratory Analysis of the Association Between Plasma Ceramide Alterations and Cognitive Dysfunction in Parkinson's Disease.CNS Neurosci Ther. 2024 Oct;30(10):e70082. doi: 10.1111/cns.70082. CNS Neurosci Ther. 2024. PMID: 39428566 Free PMC article.
-
High-throughput screening for small-molecule stabilizers of misfolded glucocerebrosidase in Gaucher disease and Parkinson's disease.Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2406009121. doi: 10.1073/pnas.2406009121. Epub 2024 Oct 10. Proc Natl Acad Sci U S A. 2024. PMID: 39388267 Free PMC article.
References
-
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. - PubMed
-
- Brady RO, Kanfer J, Shapiro D. The Metabolism of Glucocerebrosides. I. Purification and Properties of a Glucocerebroside-Cleaving Enzyme from Spleen Tissue. J Biol Chem. 1965;240:39–43. - PubMed
-
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
-
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous


