The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve-/- embryos but normality of PIKfyve+/- mice
- PMID: 21349843
- PMCID: PMC3075686
- DOI: 10.1074/jbc.M111.222364
The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve-/- embryos but normality of PIKfyve+/- mice
Abstract
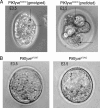
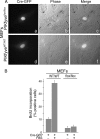
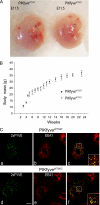
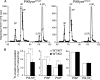
Gene mutations in the phosphoinositide-metabolizing enzymes are linked to various human diseases. In mammals, PIKfyve synthesizes PtdIns(3,5)P(2) and PtdIns5P lipids that regulate endosomal trafficking and responses to extracellular stimuli. The consequence of pikfyve gene ablation in mammals is unknown. To clarify the importance of PIKfyve and PIKfyve lipid products, in this study, we have characterized the first mouse model with global deletion of the pikfyve gene using the Cre-loxP approach. We report that nearly all PIKfyve(KO/KO) mutant embryos died before the 32-64-cell stage. Cultured fibroblasts derived from PIKfyve(flox/flox) embryos and rendered pikfyve-null by Cre recombinase expression displayed severely reduced DNA synthesis, consistent with impaired cell division causing early embryo lethality. The heterozygous PIKfyve(WT/KO) mice were born at the expected Mendelian ratio and developed into adulthood. PIKfyve(WT/KO) mice were ostensibly normal by several other in vivo, ex vivo, and in vitro criteria despite the fact that their levels of the PIKfyve protein and in vitro enzymatic activity in cells and tissues were 50-55% lower than those of wild-type mice. Consistently, steady-state levels of the PIKfyve products PtdIns(3,5)P(2) and PtdIns5P selectively decreased, but this reduction (35-40%) was 10-15% less than that expected based on PIKfyve protein reduction. The nonlinear decrease of the PIKfyve protein versus PIKfyve lipid products, the potential mechanism(s) discussed herein, may explain how one functional allele in PIKfyve(WT/KO) mice is able to support the demands for PtdIns(3,5)P(2)/PtdIns5P synthesis during life. Our data also shed light on the known human disorder linked to PIKFYVE mutations.
Figures



Similar articles
-
Functional dissociation between PIKfyve-synthesized PtdIns5P and PtdIns(3,5)P2 by means of the PIKfyve inhibitor YM201636.Am J Physiol Cell Physiol. 2012 Aug 15;303(4):C436-46. doi: 10.1152/ajpcell.00105.2012. Epub 2012 May 23. Am J Physiol Cell Physiol. 2012. PMID: 22621786 Free PMC article.
-
Class III PI 3-kinase is the main source of PtdIns3P substrate and membrane recruitment signal for PIKfyve constitutive function in podocyte endomembrane homeostasis.Biochim Biophys Acta. 2015 May;1853(5):1240-50. doi: 10.1016/j.bbamcr.2015.01.008. Epub 2015 Jan 22. Biochim Biophys Acta. 2015. PMID: 25619930 Free PMC article.
-
Unexpected severe consequences of Pikfyve deletion by aP2- or Aq-promoter-driven Cre expression for glucose homeostasis and mammary gland development.Physiol Rep. 2016 Jun;4(11):e12812. doi: 10.14814/phy2.12812. Physiol Rep. 2016. PMID: 27273882 Free PMC article.
-
PIKfyve: Partners, significance, debates and paradoxes.Cell Biol Int. 2008 Jun;32(6):591-604. doi: 10.1016/j.cellbi.2008.01.006. Epub 2008 Jan 25. Cell Biol Int. 2008. PMID: 18304842 Free PMC article. Review.
-
PIKfyve and its Lipid products in health and in sickness.Curr Top Microbiol Immunol. 2012;362:127-62. doi: 10.1007/978-94-007-5025-8_7. Curr Top Microbiol Immunol. 2012. PMID: 23086417 Review.
Cited by
-
A PIKfyve modulator combined with an integrated stress response inhibitor to treat lysosomal storage diseases.Proc Natl Acad Sci U S A. 2024 Aug 20;121(34):e2320257121. doi: 10.1073/pnas.2320257121. Epub 2024 Aug 16. Proc Natl Acad Sci U S A. 2024. PMID: 39150784
-
Selective Termination of Autophagy-Dependent Cancers.Cells. 2024 Jun 25;13(13):1096. doi: 10.3390/cells13131096. Cells. 2024. PMID: 38994949 Free PMC article. Review.
-
Inhibition of PIKfyve Leads to Lysosomal Disorders via Dysregulation of mTOR Signaling.Cells. 2024 May 30;13(11):953. doi: 10.3390/cells13110953. Cells. 2024. PMID: 38891085 Free PMC article.
-
A Novel Interaction between Chemokine and Phosphoinositide Signaling in Metastatic Prostate Cancer.Med Res Arch. 2023 Jul;11(7.1):10.18103/mra.v11i7.1.4020. doi: 10.18103/mra.v11i7.1.4020. Epub 2023 Jul 6. Med Res Arch. 2023. PMID: 38239314 Free PMC article.
-
Ablation of OCT4 function in cattle embryos by double electroporation of CRISPR-Cas for DNA and RNA targeting (CRISPR-DART).PNAS Nexus. 2023 Oct 20;2(11):pgad343. doi: 10.1093/pnasnexus/pgad343. eCollection 2023 Nov. PNAS Nexus. 2023. PMID: 37954164 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials


