Human RSPO1/R-spondin1 is expressed during early ovary development and augments β-catenin signaling
- PMID: 21297984
- PMCID: PMC3030573
- DOI: 10.1371/journal.pone.0016366
Human RSPO1/R-spondin1 is expressed during early ovary development and augments β-catenin signaling
Abstract
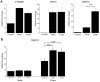
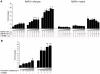
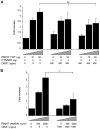
Human testis development starts from around 42 days post conception with a transient wave of SRY expression followed by up-regulation of testis specific genes and a distinct set of morphological, paracrine and endocrine events. Although anatomical changes in the ovary are less marked, a distinct sub-set of ovary specific genes are also expressed during this time. The furin-domain containing peptide R-spondin1 (RSPO1) has recently emerged as an important regulator of ovary development through up-regulation of the WNT/β-catenin pathway to oppose testis formation. Here, we show that RSPO1 is upregulated in the ovary but not in the testis during critical early stages of gonad development in humans (between 6-9 weeks post conception), whereas the expression of the related genes WNT4 and CTNNB1 (encoding β catenin) is not significantly different between these tissues. Furthermore, reduced R-spondin1 function in the ovotestis of an individual (46,XX) with a RSPO1 mutation leads to reduced β-catenin protein and WNT4 mRNA levels, consistent with down regulation of ovarian pathways. Transfection of wild-type RSPO1 cDNA resulted in weak dose-dependent activation of a β-catenin responsive TOPFLASH reporter (1.8 fold maximum), whereas co-transfection of CTNNB1 (encoding β-catenin) with RSPO1 resulted in dose-dependent synergistic augmentation of this reporter (approximately 10 fold). Furthermore, R-spondin1 showed strong nuclear localization in several different cell lines. Taken together, these data show that R-spondin1 is upregulated during critical stages of early human ovary development and may function as a tissue-specific amplifier of β-catenin signaling to oppose testis determination.
Conflict of interest statement
Figures

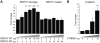
Similar articles
-
R-spondin1 signaling pathway is required for both the ovarian and testicular development in a teleosts, Nile tilapia (Oreochromis niloticus).Gen Comp Endocrinol. 2016 May 1;230-231:177-85. doi: 10.1016/j.ygcen.2016.04.001. Epub 2016 Apr 1. Gen Comp Endocrinol. 2016. PMID: 27044511
-
Cloning and expression of R-Spondin1 in different vertebrates suggests a conserved role in ovarian development.BMC Dev Biol. 2008 Jul 24;8:72. doi: 10.1186/1471-213X-8-72. BMC Dev Biol. 2008. PMID: 18651984 Free PMC article.
-
R-spondin1, WNT4, and the CTNNB1 signaling pathway: strict control over ovarian differentiation.Reproduction. 2014 Dec;148(6):R97-110. doi: 10.1530/REP-14-0177. Epub 2014 Sep 3. Reproduction. 2014. PMID: 25187620 Review.
-
WNT4 and RSPO1 together are required for cell proliferation in the early mouse gonad.Development. 2012 Dec 1;139(23):4461-72. doi: 10.1242/dev.078972. Epub 2012 Oct 24. Development. 2012. PMID: 23095882
-
Molecular mechanisms underlying female sex determination--antagonism between female and male pathway.Folia Biol (Krakow). 2009;57(3-4):105-13. doi: 10.3409/fb57_3-4.105-113. Folia Biol (Krakow). 2009. PMID: 19777952 Review.
Cited by
-
Female-Biased Expression of R-spondin 1 in Chicken Embryonic Gonads Is Estrogen-Dependent.Animals (Basel). 2023 Jul 7;13(13):2240. doi: 10.3390/ani13132240. Animals (Basel). 2023. PMID: 37444038 Free PMC article.
-
Loss of LGR4/GPR48 causes severe neonatal salt wasting due to disrupted WNT signaling altering adrenal zonation.J Clin Invest. 2023 Feb 15;133(4):e164915. doi: 10.1172/JCI164915. J Clin Invest. 2023. PMID: 36538378 Free PMC article.
-
Potential antagonistic relationship of fgf9 and rspo1 genes in WNT4 pathway to regulate the sex differentiation in Chinese giant salamander (Andrias davidianus).Front Mol Biosci. 2022 Sep 20;9:974348. doi: 10.3389/fmolb.2022.974348. eCollection 2022. Front Mol Biosci. 2022. PMID: 36203875 Free PMC article.
-
Becoming female: Ovarian differentiation from an evolutionary perspective.Front Cell Dev Biol. 2022 Sep 7;10:944776. doi: 10.3389/fcell.2022.944776. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158204 Free PMC article. Review.
-
Early Gonadal Development and Sex Determination in Mammal.Int J Mol Sci. 2022 Jul 6;23(14):7500. doi: 10.3390/ijms23147500. Int J Mol Sci. 2022. PMID: 35886859 Free PMC article. Review.
References
-
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. - PubMed
-
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. - PubMed
-
- Mendonca BB, Domenice S, Arnhold IJ, Costa EM. 46,XY disorders of sex development (DSD). Clin Endocrinol (Oxf) 2009;70:173–187. - PubMed
-
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. - PubMed
-
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


