Making heads or tails of phospholipids in mitochondria
- PMID: 21220505
- PMCID: PMC3019561
- DOI: 10.1083/jcb.201006159
Making heads or tails of phospholipids in mitochondria
Abstract
Mitochondria are dynamic organelles whose functional integrity requires a coordinated supply of proteins and phospholipids. Defined functions of specific phospholipids, like the mitochondrial signature lipid cardiolipin, are emerging in diverse processes, ranging from protein biogenesis and energy production to membrane fusion and apoptosis. The accumulation of phospholipids within mitochondria depends on interorganellar lipid transport between the endoplasmic reticulum (ER) and mitochondria as well as intramitochondrial lipid trafficking. The discovery of proteins that regulate mitochondrial membrane lipid composition and of a multiprotein complex tethering ER to mitochondrial membranes has unveiled novel mechanisms of mitochondrial membrane biogenesis.
Figures

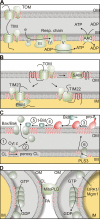
Similar articles
-
Intramitochondrial phospholipid trafficking.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Jan;1862(1):81-89. doi: 10.1016/j.bbalip.2016.08.006. Epub 2016 Aug 16. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 27542541 Review.
-
Mitochondrial lipid trafficking.Trends Cell Biol. 2014 Jan;24(1):44-52. doi: 10.1016/j.tcb.2013.07.011. Epub 2013 Sep 1. Trends Cell Biol. 2014. PMID: 24001776 Review.
-
Intramitochondrial transport of phosphatidic acid in yeast by a lipid transfer protein.Science. 2012 Nov 9;338(6108):815-8. doi: 10.1126/science.1225625. Epub 2012 Oct 4. Science. 2012. PMID: 23042293
-
Phospholipid ebb and flow makes mitochondria go.J Cell Biol. 2020 Aug 3;219(8):e202003131. doi: 10.1083/jcb.202003131. J Cell Biol. 2020. PMID: 32614384 Free PMC article. Review.
-
Mitochondrial contact sites as platforms for phospholipid exchange.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Jan;1862(1):69-80. doi: 10.1016/j.bbalip.2016.07.010. Epub 2016 Jul 29. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 27477677 Review.
Cited by
-
EMC1 Is Required for the Sarcoplasmic Reticulum and Mitochondrial Functions in the Drosophila Muscle.Biomolecules. 2024 Oct 5;14(10):1258. doi: 10.3390/biom14101258. Biomolecules. 2024. PMID: 39456191 Free PMC article.
-
Phospholipid Scramblase Activity of VDAC Dimers: New Implications for Cell Death, Autophagy and Ageing.Biomolecules. 2024 Sep 26;14(10):1218. doi: 10.3390/biom14101218. Biomolecules. 2024. PMID: 39456151 Free PMC article. Review.
-
Organelle Communication with the Nucleus.Results Probl Cell Differ. 2024;73:3-23. doi: 10.1007/978-3-031-62036-2_1. Results Probl Cell Differ. 2024. PMID: 39242372 Review.
-
Daily Light Onset and Plasma Membrane Tethers Regulate Mitochondria Redistribution within the Retinal Pigment Epithelium.Cells. 2024 Jun 25;13(13):1100. doi: 10.3390/cells13131100. Cells. 2024. PMID: 38994953 Free PMC article.
-
Mitochondria-associated endoplasmic reticulum membranes tethering protein VAPB-PTPIP51 protects against ischemic stroke through inhibiting the activation of autophagy.CNS Neurosci Ther. 2024 Apr;30(4):e14707. doi: 10.1111/cns.14707. CNS Neurosci Ther. 2024. PMID: 38584329 Free PMC article.
References
-
- Achleitner G., Gaigg B., Krasser A., Kainersdorfer E., Kohlwein S.D., Perktold A., Zellnig G., Daum G. 1999. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur. J. Biochem. 264:545–553 10.1046/j.1432-1327.1999.00658.x - DOI - PubMed
-
- Ardail D., Privat J.P., Egret-Charlier M., Levrat C., Lerme F., Louisot P. 1990. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 265:18797–18802 - PubMed
-
- Ardail D., Lerme F., Louisot P. 1991. Involvement of contact sites in phosphatidylserine import into liver mitochondria. J. Biol. Chem. 266:7978–7981 - PubMed
-
- Ardail D., Gasnier F., Lermé F., Simonot C., Louisot P., Gateau-Roesch O. 1993. Involvement of mitochondrial contact sites in the subcellular compartmentalization of phospholipid biosynthetic enzymes. J. Biol. Chem. 268:25985–25992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


